Table of Contents
Credits
Hosts: Ella Dzora, Sara Dong
Guest: Jason Brophy
Writing: Justin Penner
Producing/Editing/Cover Art/Infographics: Sara Dong
Our Guests
Guest Consultant:
Dr. Jason Brophy

Dr. Jason Brophy is a pediatric infectious diseases specialist and researcher at the Children’s Hospital of Eastern Ontario, and an Associate Professor of Pediatrics at the University of Ottawa. His research and clinical interests are pediatric HIV and congenital infections. He also works as a Pediatric HIV Clinical Advisor with the Clinton Health Access Initiative (CHAI), supporting the uptake of optimal pediatric HIV care in West-Central Africa and Southeast Asia.
Guest Co-Host:
Dr. Ella Dzora

Dr Ella Dzora is a paediatric Registrar in South Yorkshire, England. She has an interest in Infectious Diseases and Global Health, particularly migrant health.
Culture
Arno Karlan
Consult Notes
Case Summary
23 day old baby admitted with seizures, intracranial calcifications, and ventriculomegaly who was diagnosed with congenital toxoplasmosis
Key Points
Some key references for the information below and reference when you have future cases!
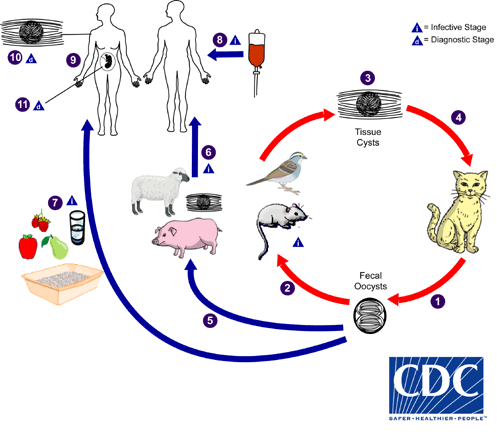
A quick microbiology refresher on Toxoplasma gondii (T.gondii)
- T.gondii is a protozoan and obligate intracellular parasite
- You can find the life cycle on the CDC website and reproduced to the right
- Unique biphasic life cycle (sexual cycle that occurs in felines + asexual cycle that can occur in other animals and humans)
- Infectious forms: tachyzoites, tissue cysts containing bradyzoites, and oocysts containing sporozoites
- The tachyzoite and corresponding host immune reaction are responsible for observed symptoms
T.gondii epidemiology
- Sources of transmission:
-
- Raw, undercooked, or cured meat or meat products
- Contaminated soil or water
- Unpasteurized milk
- Contaminated seafood
- Transplant, transfusion
- Congenital (more on this in the toxoplasma & pregnancy section later)
-
- The seroprevalence of T.gondii infection varies by geography and socioeconomic strata of the population
-
- The overall T.gondii seropositivity rate in the US among women 15-44 years of age has been estimated ~7%
-
- The highest reported rates of infection have been reported in Europe, Central America, Brazil, and Central Africa
-
- In some European countries, the rates of congenital toxo have declined in setting of aggressive screening practices and prevention programs
-
- A few references in addition to the key resources at the top of the post:
-
- Berger F, Goulet V, Le Strat Y, Desenclos JC. Toxoplasmosis among pregnant women in France: risk factors and change of prevalence between 1995 and 2003. Rev Epidemiol Sante Publique. 2009;57(4):241-248. doi:10.1016/j.respe.2009.03.006
- Dubey JP, Murata FHA, Cerqueira-Cézar CK, Kwok OCH, Villena I. Congenital toxoplasmosis in humans: an update of worldwide rate of congenital infections [published correction appears in Parasitology. 2021 Nov;148(13):1716]. Parasitology. 2021;148(12):1406-1416. doi:10.1017/S0031182021001013
- Picone O, Fuchs F, Benoist G, et al. Toxoplasmosis screening during pregnancy in France: Opinion of an expert panel for the CNGOF. J Gynecol Obstet Hum Reprod. 2020;49(7):101814. doi:10.1016/j.jogoh.2020.101814
-
What are the clinical manifestations of congenital toxoplasmosis?
- Most newborns will be asymptomatic.
- The “classic triad” of congenital toxoplasmosis: chorioretinitis, cerebral calcification, hydrocephalus
- These are highly suggestive but this triad is only present in <10% of cases!
- ~10-30% of infected newborns will have clinically apparent signs and symptoms at birth or early infancy
- Postnatal presentations have been grouped as:
- Severe neonatal disease (usually from primary maternal infection during first trimester)
- Mild to moderate disease that is clinically apparent within the first few months of life
- Subclinical infection
- Late sequelae of undiagnosed infection that may become apparent later in infancy, childhood, or adolescence
- Signs/symptoms
- Chorioretinitis = the most common late manifestation
- Typical lesion is focal necrotizing retinitis
- Associated findings may include: microphthalmia, strabismus, cataracts, nystagmus
- Incidence of new onset retinal lesions in untreated children approaches 70-90% (and up to 25% of those who were treated) >> and the risk extends into adulthood
- Intracranial calcifications
- Hydrocephalus
- Meningoencephalitis with CSF abnormalities
- Microcephaly
- Seizures
- Hearing impairment or loss
- Petechial rash
- Fever
- Jaundice
- Generalized lymphadenopathy
- Hepatosplenomegaly
- Pneumonitis
- Thrombocytopenia
- Anemia
- Jaundice
- Chorioretinitis = the most common late manifestation
- Some severely affected fetuses/infants with disseminated congenital toxoplasmosis die in utero or within a few days of birth
- Visual or hearing impairment, learning disabilities, or severe developmental delay will become apparent later in life in large proportion of congenitally infected infants
- A few more references in addition to the key resources at the top of the post:
- Tamma P. Toxoplasmosis. Pediatr Rev. 2007;28(12):470-471. doi:10.1542/pir.28-12-470
- McLeod R, Boyer K, Karrison T, et al. Outcome of treatment for congenital toxoplasmosis, 1981-2004: the National Collaborative Chicago-Based, Congenital
- Toxoplasmosis Study. Clin Infect Dis. 2006;42(10):1383-1394. doi:10.1086/501360
- McAuley J, Boyer KM, Patel D, et al. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago Collaborative Treatment Trial [published correction appears in Clin Infect Dis 1994 Oct;19(4):820]. Clin Infect Dis. 1994;18(1):38-72. doi:10.1093/clinids/18.1.38
- Sever JL, Ellenberg JH, Ley AC, et al. Toxoplasmosis: maternal and pediatric findings in 23,000 pregnancies. Pediatrics. 1988;82(2):181-192.
- Wilson CB, Remington JS, Stagno S, Reynolds DW. Development of adverse sequelae in children born with subclinical congenital Toxoplasma infection. Pediatrics. 1980;66(5):767-774.
Toxoplasmosis in pregnancy
- Mothers are not screened routinely for toxoplasmosis during pregnancy in the US
-
- To be effective, screening would need to be done frequently to detect and in turn treat asymptomatic maternal infection (ideally within 3 weeks) >> but frequent rescreenign is costly and bothersome for patients
-
- Incidence of acute primary T.gondii infection during pregnancy in US is estimated to be 0.2-1.1/1000 women on basis of data from MA and NH, which are the only 2 states in the US with universal newborn toxo screening
- Congenital transmission occurs in most cases as a result of acute primary maternal infection acquired during pregnancy or within 3 months prior to conception
-
- In utero infection rarely can occur as a result of reactivated disease in latently infected immunocompromised woman in absence of prophylaxis
- Rarely, congenital toxoplasmosis is acquired from immunocompetent pregnant woman reinfected with different T.gondii strain
-
- Critical illness is more likely to be severe in newborn when infection occurs in first trimester and is not treated during gestation
-
- As gestational age increases, the risk of infection in fetus increases, but severity of disease decreases (those infected in 2nd and 3rd trimesters typically have mild or subclinical disease at birth)
-
- Risk factors for maternal to fetal transmission
-
- Maternal infection at advanced gestational age
- High parasite load
- Maternal parasite source (higher risk if sporozoites in oocysts vs. bradyzoites in tissue cysts)
- High virulence T.gondii strain
- Maternal immunocompromise
- Rico-Torres CP, Vargas-Villavicencio JA, Correa D. Is Toxoplasma gondii type related to clinical outcome in human congenital infection? Systematic and critical review. Eur J Clin Microbiol Infect Dis. 2016;35(7):1079-1088. doi:10.1007/s10096-016-2656-2
-
- More on maternal treatment in the prevention section
- During pregnancy, PCR assay of amniotic fluid is method of choice to confirm fetal infection
-
- Fetal US can assess for anatomic abnormalities
- Examination of placenta by histology and PCR assay may provide information but is not sufficient for diagnosis
-
What is involved in the initial evaluation of the newborn with suspected congenital toxoplasmosis?
- Review maternal history and serology if available
- Comprehensive physical exam
-
- See the clinical manifestations section above for more
- The exam is going to be normal in most cases, but make sure to look for fever, jaundice, hepatosplenomegaly, neurologic changes, and other symptoms
-
- Ophthalmology exam to look for chorioretinitis or other changes
- Newborn hearing screening
- LP for CSF: cell count and diff, glucose, protein, T.gondii PCR
-
- CSF may demonstrate: elevated protein, hypoglycorrhachia, eosinophilia
-
- In addition to CSF, various other samples can be sent for T.gondii PCR (at reference lab): urine, whole blood, vitreous fluid, BAL, cord blood, placenta
- Neuroimaging which may demonstrate cerebral calcification
-
- Head US: doesn’t capture calcifications as wel but often used in regions where symptomatic congenital toxo is low
- Head CT: most sensitive for calcifications and can reveal brain abnormalities (like ventriculomegaly and hydrocephalus) when ultrasound studies are normally
-
- Generally preferred
-
- Brain MRI (but requires sedation)
-
- Serology (paired baby and maternal serology testing) – more detail in the next section
-
- Toxoplasma IgG
- Toxoplasma IgM (ELISA or ISAGA)
- Toxoplasma IgA (ELISA or ISAGA)
-
- Other testing and screening
-
- CBC with differential
- AST, ALT, total and direct bilirubin
- UA and Cr
- G6PD deficiency evaluation
- Evaluation for other infections on the differential (many congenital infections have clinical overlap!)
-
- Abdominal US can be considered to evaluate for hepatosplenomegaly or intrahepatic calcifications
Let's focus on the Toxoplasma serology testing in more detail
- So initial serology testing includes blood for IgG, IgM, IgA >> an accurate serologic diagnosis requires testing of blood from both infant and mother
- Send as soon as possible after birth (however repeat testing may be necessary to exclude false positives)
- Toxoplasma IgG
- Doesn’t tell us the difference between maternal and infant infection in the newborn period
- Toxoplasma-specific IgM
- Indicates congenital infection if not contaminated with maternal blood
- Toxoplasma-specific IgA
- IgA is not always necessary but often useful and performed in conjunction with IgM at some reference labs
- Especially useful if IgG and IgM assays are indeterminate
- Confirmatory testing for pregnant women with suspected acute Toxo infection or neonates with suspected congenital toxo should be sent to a reference lab, which are more sensitive than commercial labs. Reference labs perform the:
- IgG ELISA or dye test
- Toxo IgM ELISA or immunosorbent agglutination assay (ISAGA)
- Toxo IgA ELISA or ISAGA
- IgG avidity
- Reference lab with special expertise: Palo Alto Medical Foundation Toxoplasma Serology Lab (RemingtonLab@sutterhealth.org)
- A summary of neonatal diagnostic test results at birth or postnatally:
- Confirmed diagnosis:
- +IgG with +IgM (after DOL5) and/or +IgA (after DOL10)
- +CSF T.gondii PCR
- ↑IgG titer during 1st yr of life or ↑titer compared to mom
- +IgG beyond 12 mo of age
- Presumed diagnosis:
- Characteristic clinical findings, +IgG, but negative IgM and IgA
- Excluded:
- -IgG, -IgM, -IgA
- -IgM, -IgA with positive IgG titer that declines over time in absence of tx
- Confirmed diagnosis:
- A summary of some of the challenges of newborn serologic testing
- Toxoplasma IgG in newborn may reflect past or current infection in mother (IgG crosses placenta)
- Fetal/newborn Ab response to T.gondii is variable. Depending on timing of maternal infection, Toxo-specific IgM may disappear before birth, may be demonstrated within first few days or life, or may be delayed in months
- Antenatal treatment may affect the serologic profile of infant. IgM usually is undetectable or reduced, along with reduction in IgA, in infants exposed to anti-Toxo therapy with pyrimethamine and sulfadiazine in utero
- Though maternal IgM and IgA Abs do not cross the placenta, small amounts can leak from the placenta which may results in low positive IgM or IgA in an uninfected infant shortly after birth
- Repeat testing might be needed in certain scenarios:
- Asymptomatic newborn infant; low suspicion for congenital toxo; initially IgG+, but IgM- and IgA-
- Follow-up IgG at 4-6 wk intervals until complete disappearance of IgG antibodies (usually within 6-12 months)
- In absence of postnatal treatment, disappearance of IgG in the infant excludes a diagnosis of congenital toxo >> transplacentally derived maternal Toxo IgG is expected to decrease by 50% per month and usually becomes undetectable by 6-12 months of life
- Asymptomatic newborn infant; positive initial IgM and/or IgA
- Repeat testing at least 10 days after birth to determine if false positive
- False positive IgM and IgA titers can occur following blood product transfusion >> repeat testing after at least 7 days after last transfusion
- Negative initial results; strong clinical suspicion
- May be false negative due to delayed antibody production or antenatal treatment
- Retest 2-4 weeks after birth and every 4 weeks until 3 months of age
- Asymptomatic newborn infant; low suspicion for congenital toxo; initially IgG+, but IgM- and IgA-
- A few more resources in addition to those at top of the blog post:
Treatment of congenital toxoplasmosis
Most cases of acquired acute T.gondii infections in immunocompetent hosts do not require therapy but they DO if infection occurs during pregnancy, there is ocular involvement, or there are severe or persistent symptoms!!
- There is not standardized practice, and treatment regimens vary across the world. Comparative data on the different treatment options is lacking. Most recommendations are in line with what is noted below though. Check out more at these treatment recommendation links:
- Maldonado YA, Read JS; COMMITTEE ON INFECTIOUS DISEASES. Diagnosis, Treatment, and Prevention of Congenital Toxoplasmosis in the United States. Pediatrics. 2017;139(2):e20163860. doi:10.1542/peds.2016-3860
- Montoya JG, Remington JS. Management of Toxoplasma gondii infection during pregnancy. Clin Infect Dis. 2008;47(4):554-566. doi:10.1086/590149
- Peyron F, L’ollivier C, Mandelbrot L, et al. Maternal and Congenital Toxoplasmosis: Diagnosis and Treatment Recommendations of a French Multidisciplinary Working Group. Pathogens. 2019;8(1):24. Published 2019 Feb 18. doi:10.3390/pathogens8010024
- Preferred regimens include oral therapy with:
- Pyrimethamine 1 mg/kg every 12 hours x 2 days, then 1 mg/kg once daily x 2-6 months, then 1 mg/kg once per day Mon/Wed/Fri to complete 12 months
- Sulfadiazine 50 mg/kg every 12 hours for 12 months
- Folinic acid (leucovorin) 10 mg/dose 3 times per week (during and up to 1 week after completing pyrimethamine)
- These are from the AAP Red Book Recommendations
- Most experts recommend one year of treatment, although some centers extend up to 2 years
- Adjunctive corticosteroids are added if (1) CSF protein ≥1 g/dL and/or (2) severe active chorioretinitis, vision threatening
- Prednisolone 0.5 mg/kg twice daily (max 20 mg/dose)
- Continue until elevated CSF protein or active chorioretinitis lesions are resolved
- **If pred is used, should be started 48-72 hrs after initiation of anti-Toxo therapy**
- Based on observational data and expert opinion
- What about infected neonates with asymptomatic congenital toxoplasmosis? These patients are managed with the same regimen used for symptomatic infants
- Treatment duration might be shorter, but should be at least 3 months and determined in discussion with congenital toxo expert
- Most evidence for therapy is from observational studies or prior HIV clinical trials, but here are a few key articles:
- McAuley J, Boyer KM, Patel D, et al. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago Collaborative Treatment Trial [published correction appears in Clin Infect Dis 1994 Oct;19(4):820]. Clin Infect Dis. 1994;18(1):38-72. doi:10.1093/clinids/18.1.38
- Guerina NG, Hsu HW, Meissner HC, et al. Neonatal serologic screening and early treatment for congenital Toxoplasma gondii infection. The New England Regional Toxoplasma Working Group. N Engl J Med. 1994;330(26):1858-1863. doi:10.1056/NEJM199406303302604
- McLeod R, Boyer K, Karrison T, et al. Outcome of treatment for congenital toxoplasmosis, 1981-2004: the National Collaborative Chicago-Based, Congenital
Monitoring
- While on medication, close safety/toxicity lab monitoring:
-
- Monitor weekly for neutropenia (but can space out later if CBCs are stable)
-
- If ANC decreases <750, frequency of folinic acid administration should be increased (to daily dosing) and pyrimethamine therapy should be temporarily held
-
- Monitor weekly for neutropenia (but can space out later if CBCs are stable)
-
- Serology monitoring every 3-6 months until 18 months of age or completion of therapy (especially important for unconfirmed but highly suspected cases)
-
- IgM typically becomes undetectable while on treatment, but commonly increases once therapy is discontinued >> the clinical significance of these rebound titers is uncertain, but likely implies immune response to treatment (not necessarily treatment failure or reactivation)
-
- Ophthalmologic evaluations should be continued at least every 3-6 months during first 3 years of life (even if initial evaluation was normal)
-
- Remember to discuss the chances of ocular re-activation later in life such as in puberty and the possible need for re-treatment
-
- Hearing evaluation
- Long-term neurologic/neurodevelopmental follow-up
Prevention
- Pregnant women and immunocompromised patients whose serostatus for Toxo infection is negative or unknown should be counseled on how to avoid activities that may expose them to infection:
-
- Decrease exposure to cat feces
-
- Avoid changing litter boxes, gardening, landscaping
- If these activities must be done:
-
- Wear gloves and wash hands immediately after
- Change cat litter daily to decrease risk of infection (oocysts are not infective during the first 1-2 days after passage)
-
- Domestic cats can be protected from infection by feeding them commercially prepared cat food and preventing them from eating undercooked meat and hunting wild rodents and birds
-
- Oral ingestion can be prevented by:
-
- Avoiding consumption of raw or undercooked meat; smoked meat and meat cured in brine; raw shellfish; raw/unpasteurized milk; untreated water
- Freezing meat to -20C for 48h before consumption
- Washing fruits and vegetables
-
- Decrease exposure to cat feces
-
- The episode also briefly touched on maternal treatment for reduction of congenital toxoplasmosis
-
- Treatment can be offered to pregnant patients diagnosed with recent T.gondii infection acquired during pregnancy
- Needs to be given as soon as possible after probable maternal infection >> the therapeutic window of opportunity is <3 wks from seroconversion
-
- This is the time for transition from acute infective tachyzoite form to dormant bradyzoite form (cysts are not susceptible to antibiotics)
-
- The regimens are based on gestational age at diagnosis
-
- First trimester (<14 wks) >>> spiramycin
- After amniocentesis or ≥14 wks >>> pyrimethamine-sulfadiazine
-
- Pyr-sulfa is superior treatment based on the available evidence (see below)
-
- You can check out a few papers related to prenatal treatment below:
-
- An overview: Mandelbrot L. Congenital toxoplasmosis: What is the evidence for chemoprophylaxis to prevent fetal infection?. Prenat Diagn. 2020;40(13):1693-1702. doi:10.1002/pd.5758
- Mandelbrot L, Kieffer F, Sitta R, et al. Prenatal therapy with pyrimethamine + sulfadiazine vs spiramycin to reduce placental transmission of toxoplasmosis: a multicenter, randomized trial. Am J Obstet Gynecol. 2018;219(4):386.e1-386.e9. doi:10.1016/j.ajog.2018.05.031
-
- The TOXOGEST French multicenter trial compared spiramycin vs pyr-sulfa in pts with toxo seroconversion when gestational age >14 wks
- 143 patients were randomized
- Transmission rates trended lower in the pyr-sulfa group (18.5% vs 30%, OR 0.53, 95% CI 0.23-1.22), and there was a greater reduction in congenital toxo when pyr-sulfa was started within 3 wks of seroconversion
- Favored pyr-sulfa over spiramycin, but didn’t prove that treatment is more effective than no treatment
- Trial was stopped early due to low recruitment and funding
-
- SYROCOT (Systematic Review on Congenital Toxoplasmosis) study group, Thiébaut R, Leproust S, Chêne G, Gilbert R. Effectiveness of prenatal treatment for congenital toxoplasmosis: a meta-analysis of individual patients’ data. Lancet. 2007;369(9556):115-122. doi:10.1016/S0140-6736(07)60072-5
-
- Meta-analysis of 20 European cohort studies (1438 pts)
- Assessed timing and type of prenatal treatment on MTCT and clinical manifestations before age 1 yr
-
-
CCC Series Art & Infographics
Goal
Listeners will be able to understand the diagnosis and management of congenital toxoplasmosis
Learning Objectives
After listening to this episode, listeners will be able to:
- Describe common features of congenital toxoplasmosis in a newborn
- Describe the recommended diagnostic evaluation for suspected congenital toxoplasmosis
- List the recommended treatment regimen as well as subsequent monitoring for children with congenital toxoplasmosis
Disclosures
Our guest (Jason Brophy) as well as Febrile podcast and hosts report no relevant financial disclosures
Citation
Brophy, J., Dzora, E., Dong, S. “#39: Curious Congenital Conundrums – Seal of Approval ”. Febrile: A Cultured Podcast. https://player.captivate.fm/episode/77847ca3-b214-43de-8524-db41081f4b3b