Table of Contents
Credits
Hosts: Emily Niehaus, Sara Dong
Guest: Laura Certain
Writing: Emily Niehaus, Laura Certain, Sara Dong
Producing/Editing/Cover Art/Infographics: Sara Dong
Our Guests
Guest Consultant
Laura Certain, MD, PhD

Laura Certain is an Assistant Professor of Medicine in the Division of Infectious Diseases and Adjunct Assistant Professor of Orthopedics at the University of Utah, and Chief of Infectious Diseases at the Salt Lake City VA Medical Center. She received her MD and PhD degrees from the University of Washington in Seattle, and her Internal Medicine residency and Infectious Disease fellowship training at Massachusetts General Hospital in Boston. After fellowship she completed a post-doctoral research fellowship studying prosthetic joint infections in an animal model, then moved to Utah in 2017 to focus more on clinical care of humans with orthopedic infections and less on mice. She is currently Vice-President of the Musculoskeletal Infection Society, a national organization of orthopedic surgeons, infectious disease physicians, and microbiologists with interests in orthopedic infections.
Guest Co-Host
Emily Niehaus, MD

Emily Niehaus completed her medical school training at Emory University School of Medicine followed by internal medicine residency at the University of Utah. Emily was a resident when she created this episode, but since recording, she has now become a brand new ID fellow at Duke University
Culture
Laura shared some books from Amor Towles: The Lincoln Highway, The Gentleman in Moscow
Emily shared Inventing Anna, which is on Netflix
Consult Notes
Consult Q
Assistance with work-up of a man with low back pain and fever
Case Summary
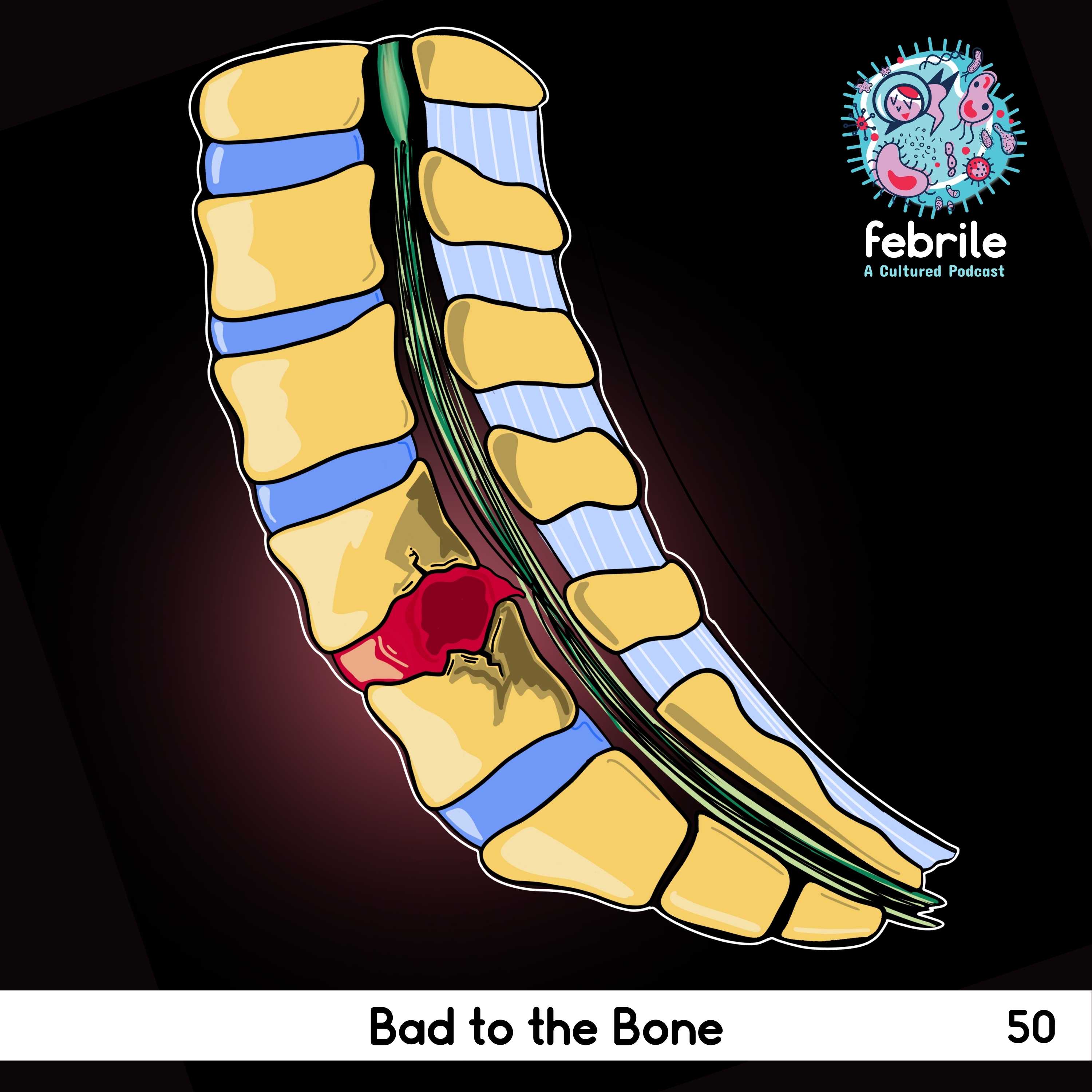
65 yo male with acute MRSA L2-L3 discitis/osteomyelitis. His course was complicated by late development of epidural fluid collection and spinal canal stenosis, which required spinal fusion/hardware placement.
Key Points
Some overview resources
- You can find the IDSA guidelines here: Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis. 2015;61(6):e26-e46. doi:10.1093/cid/civ482
- A general overview of osteomyelitis: Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364(9431):369-379. doi:10.1016/S0140-6736(04)16727-5
- A NEJM Clinical Practice Case: Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med. 2010;362(11):1022-1029. doi:10.1056/NEJMcp0910753
- Boody BS, Jenkins TJ, Maslak J, Hsu WK, Patel AA. Vertebral Osteomyelitis and Spinal Epidural Abscess: An Evidence-based Review. J Spinal Disord Tech. 2015;28(6):E316-E327. doi:10.1097/BSD.0000000000000294
Let’s start with the basics of vertebral osteomyelitis
- Vertebral osteomyelitis is most commonly the result of hematogenous seeding of one or more vertebral bodies from a distant focus of infection
- Organisms can produce a spontaneous local suppurative infection, which may be facilitated by prior bone trauma / disrupted architecture
- Segmental arteries supplying the vertebrae usually split to supply 2 adjacent end plates >> which is why you often see bone destruction in 2 adjacent vertebral bodies and their intervertebral disc
- Infection can also occur via:
- Direct inoculation: following surgery, injection of the disc space, or trauma
- Contiguous spread from adjacent soft tissue infection (such as aorta, esophagus, bowel)
- Vertebral osteomyelitis and discitis may occur together or independently
- Occurs most commonly in adults, particularly older adults >50
What are potential risk factors for vertebral osteomyelitis?
- Consider what might lead to a transient bacteremia, such as:
- Injection drug use
- Maybe they had a recent hospitalization with other infection or cathether placement
- Infective endocarditis
- Sites of prior damage to the bone, such as:
- Degenerative spine disease
- Prior spinal surgery
- General medical comorbidities that decrease the immune system:
- Diabetes mellitus
- Corticosteroid therapy
- Liver disease
- Immunocompromised state
- Then of course, pathogen-specific risk factors.
- Brucella and TB make up a large proportion of cases in other locations. Here is the study from Turkey that Laura referenced on the show: Eren Gök S, Kaptanoğlu E, Celikbaş A, et al. Vertebral osteomyelitis: clinical features and diagnosis. Clin Microbiol Infect. 2014;20(10):1055-1060. doi:10.1111/1469-0691.12653
- This study demonstrated 45% Brucella, 29% TB, 26% pyogenic bacteria. In this study, a positive PPD had sensitivity of 0.66, specificity 0.97 for diagnosing TB vertebral osteomyelitis. As Laura discussed, it’s worth noting we often discourage use of testing meant for LTBI in trying to diagnose active TB
- Endemic fungi, especially coccidiomycosis, is also a consideration if there are risk factors
- Brucella and TB make up a large proportion of cases in other locations. Here is the study from Turkey that Laura referenced on the show: Eren Gök S, Kaptanoğlu E, Celikbaş A, et al. Vertebral osteomyelitis: clinical features and diagnosis. Clin Microbiol Infect. 2014;20(10):1055-1060. doi:10.1111/1469-0691.12653
What is the usual presentation for discitis and osteomyelitis?
- New or worsening back or neck pain! Typically localized to the infected disc space
- Can be exacerbated by movement or percussion; Also may radiate
- This pain may also be insidious with a slow progression in symptoms over weeks to months
- In addition, if the infection extends posteriorly into the epidural space, might see features of epidural abscess such as severe back pain with radiculopathy, then motor weakness and sensory changes (including loss of bowel and bladder control; loss of perineal sensation)
- Fever only present maybe half the time
- WBC count may be elevated or normal
- Inflammatory markers – fairly sensitive but not specific, and may not be as high if the infection is with lower virulence organisms
- Carragee EJ, Kim D, van der Vlugt T, Vittum D. The clinical use of erythrocyte sedimentation rate in pyogenic vertebral osteomyelitis. Spine (Phila Pa 1976). 1997;22(18):2089-2093. doi:10.1097/00007632-199709150-00005
- Unkila-Kallio L, Kallio MJ, Eskola J, Peltola H. Serum C-reactive protein, erythrocyte sedimentation rate, and white blood cell count in acute hematogenous osteomyelitis of children. Pediatrics. 1994;93(1):59-62.
- Ghassibi M, Yen TC, Harris S, Si Z, Leary E, Choma TJ. Responsiveness of routine diagnostic tests for vertebral osteomyelitis may be influenced by the infecting organism. Spine J. 2021;21(9):1479-1488. doi:10.1016/j.spinee.2021.04.001
Microbiology of vertebral osteomyelitis
- Typically infections will be monomicrobial
- Staphylococcal species are the most common cause (>50%)
- Streptococci (including group B, groups C/G, viridans group, milleri group) and enteric Gram negatives also make up a sizable portion
- Pseudomonas aeruginosa, coagulase negative staph and Candida can be seen in particular with intravascular access, sepsis, or injection drug use
- Laura also discussed some of the items that we see less commonly in the US. There are pathogens that should be on the differential in certain patients with epidemiologic risk factors:
- Tuberculosis (Pott’s Disease)
- Brucellosis
- Coccidioides and endemic fungi
- Burkholderia pseudomallei (meliodosis)
Evaluation of possible vertebral discitis or osteomyelitis
- Diagnosis is based on positive culture from a biopsy, but diagnosis is also inferred in some settings such as:
- Typical clinical and radiographic findings and positive blood cultures with a likely pathogen
- Histopathologic findings consistent with infection in absence of positive culture data
- Suggestive clinical and typical radiographic findings with persistently elevated inflammatory markers
- First, as always, careful history and physical exam. Pay particular attention to neurologic exam
- Blood cultures should always be drawn prior to starting antibiotics!
- As mentioned above, if blood cultures are positive for an organism known to cause vertebral osteomyelitis, then likely don’t need further work-up (such as S.aureus, S.lugdunensis, certain gram negative organisms) [this is what IDSA guidelines suggest]
- Send inflammatory markers (CRP and ESR)
- If patients have indications, other diagnostic tests might include: Brucella serologies, endemic fungi testing, PPD
- Spinal imaging:
- MRI is the most sensitive for vertebral osteomyelitis and epidural abscess
- It is often advisable to image the entire spine, as “skip” lesions happen ~5% of the time
- The next step for diagnosis would be image-guided biopsy
- If possible, it is best to hold antibiotics in a stable patient until microbiologic diagnosis is made. The key exceptions are in the setting of neurologic compromise and sepsis.
- How much a single dose of antibiotics affects the yield varies between studies
- Saravolatz LD 2nd, Labalo V, Fishbain J, Szpunar S, Johnson LB. Lack of effect of antibiotics on biopsy culture results in vertebral osteomyelitis. Diagn Microbiol Infect Dis. 2018;91(3):273-274. doi:10.1016/j.diagmicrobio.2018.02.017
- Weihe R, Taghlabi K, Lowrance M, et al. Culture Yield in the Diagnosis of Native Vertebral Osteomyelitis: A Single Tertiary Center Retrospective Case Series With Literature Review. Open Forum Infect Dis. 2022;9(3):ofac026. Published 2022 Feb 7. doi:10.1093/ofid/ofac026
- How much a single dose of antibiotics affects the yield varies between studies
- Send sample for bacterial (aerobic and anaerobic), fungal, AFB cultures + histopathology
- Unfortunately, sensitivity is not great. There is a big range in the literature but you have about a 50/50 chance
- Biopsies from infected disc spaces may have higher yield than bone biopsies
- If possible, it is best to hold antibiotics in a stable patient until microbiologic diagnosis is made. The key exceptions are in the setting of neurologic compromise and sepsis.
- If your cultures and first biopsy were unrevealing and the clinical suspicion remains high, you have a few choices:
- You can try to convince the patient and radiologist to try again vs asking a surgeon about an open biopsy
- Or you can treat empirically (which is often what is going to have in practice)
- Throughout the work-up, the patient needs close monitoring for progression of neurologic signs. At any point, a patient may require evaluation by surgery. Important times to ensure surgical evaluation include:
- Neurologic deficits, especially if progressive
- Radiographic evidence of epidural or paravertebral abscess
- Cord compression or spinal instability
- Progressive deformity despite adequate antimicrobial therapy
- Persistent or recurrent bloodstream infection or worsening pain despite antimicrobial therapy
- A few additional resources:
- An HS, Seldomridge JA. Spinal infections: diagnostic tests and imaging studies. Clin Orthop Relat Res. 2006;444:27-33. doi:10.1097/01.blo.0000203452.36522.97
- Chang CY, Simeone FJ, Nelson SB, Taneja AK, Huang AJ. Is Biopsying the Paravertebral Soft Tissue as Effective as Biopsying the Disk or Vertebral Endplate? 10-Year Retrospective Review of CT-Guided Biopsy of Diskitis-Osteomyelitis. AJR Am J Roentgenol. 2015;205(1):123-129. doi:10.2214/AJR.14.13545
- Chong BSW, Brereton CJ, Gordon A, Davis JS. Epidemiology, Microbiological Diagnosis, and Clinical Outcomes in Pyogenic Vertebral Osteomyelitis: A 10-year Retrospective Cohort Study. Open Forum Infect Dis. 2018;5(3):ofy037. Published 2018 Mar 10. doi:10.1093/ofid/ofy037
How should we treat native pyogenic vertebral osteomyelitis?
- Most experts will routinely treat a minimum of 6 weeks, with careful review to determine if longer therapy is required
- Here are key studies for you to check out
- Bernard L, Dinh A, Ghout I, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet. 2015;385(9971):875-882. doi:10.1016/S0140-6736(14)61233-2
- A RCT that demonstrated six weeks was non-inferior to twelve weeks of antibiotics for pyogenic vertebral osteomyelitis (based on proportion of patients cured at one year)
- Number of patients with abscesses was low
- Most common oral regimen was fluoroquinolone with rifampin
- Park KH, Cho OH, Lee JH, et al. Optimal Duration of Antibiotic Therapy in Patients With Hematogenous Vertebral Osteomyelitis at Low Risk and High Risk of Recurrence. Clin Infect Dis. 2016;62(10):1262-1269. doi:10.1093/cid/ciw098
- A retrospective review. Found independent risk factors conferring increased likelihood of relapse in patients treated <8 wks were presence of undrained paravertebral abscess and MRSA infection
- This study showed prolonged duration of antibiotics in these high risk patients led to lower likelihood of recurrence, but would note this was an observational study
- Bernard L, Dinh A, Ghout I, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet. 2015;385(9971):875-882. doi:10.1016/S0140-6736(14)61233-2
- When can we switch to PO?
- Based on OVIVA, probably anytime assuming there is an antibiotics with good oral availability and there is reliable absorption of the med
- An additional paper: Babouee Flury B, Elzi L, Kolbe M, et al. Is switching to an oral antibiotic regimen safe after 2 weeks of intravenous treatment for primary bacterial vertebral osteomyelitis?. BMC Infect Dis. 2014;14:226. Published 2014 Apr 27. doi:10.1186/1471-2334-14-226
How should we monitor these patients in follow-up?
- Monitor for clinical signs of soft tissue extension, paraspinal abscess, or cord compression
- Trending ESR and CRP can give you some sense of who is responding to therapy, but it’s not clear what to do if the values don’t decrease
- If they remain high at four weeks, the patient may be more likely to fail therapy
- Yoon SH, Chung SK, Kim KJ, Kim HJ, Jin YJ, Kim HB. Pyogenic vertebral osteomyelitis: identification of microorganism and laboratory markers used to predict clinical outcome. Eur Spine J. 2010;19(4):575-582. doi:10.1007/s00586-009-1216-1
- Carragee EJ, Kim D, van der Vlugt T, Vittum D. The clinical use of erythrocyte sedimentation rate in pyogenic vertebral osteomyelitis. Spine (Phila Pa 1976). 1997;22(18):2089-2093. doi:10.1097/00007632-199709150-00005
- Khan MH, Smith PN, Rao N, Donaldson WF. Serum C-reactive protein levels correlate with clinical response in patients treated with antibiotics for wound infections after spinal surgery. Spine J. 2006;6(3):311-315. doi:10.1016/j.spinee.2005.07.006
- In general, repeat imaging is not advised – people get better long before their imaging does!
- MRI/CT/plain films may appear to worsen for several weeks after initiation of antibiotics therapy (that will ultimately be successful)
- Kowalski TJ, Layton KF, Berbari EF, et al. Follow-up MR imaging in patients with pyogenic spine infections: lack of correlation with clinical features. AJNR Am J Neuroradiol. 2007;28(4):693-699.
- Carragee EJ. The clinical use of magnetic resonance imaging in pyogenic vertebral osteomyelitis. Spine (Phila Pa 1976). 1997;22(7):780-785. doi:10.1097/00007632-199704010-00015
- The times to consider imaging would include:
- Lack of clinical improvement, to evaluate for presence of abscess (in need of drainage) or
- To detect spinal instability amenable to surgical evaluation
In Emily’s case, the patient failed the initial course and had hardware implanted. How should we manage these patients with spinal hardware associated infection?
- Laura typically does at least 8 weeks of antibiotics in these patients
- Based on retrospective study of patients with hematogenous vertebral osteomyelitis who had hardware placed into the infected space >> those that got >8 wks of antibiotics had lower risk of recurrence: Park KH, Cho OH, Lee YM, et al. Therapeutic outcomes of hematogenous vertebral osteomyelitis with instrumented surgery. Clin Infect Dis. 2015;60(9):1330-1338. doi:10.1093/cid/civ066
- What about rifampin? There is not enough data to say whether or not adding rifampin is beneficial in this setting. Anecdotally, many ID doctors will add if there are no contraindications to it
We discussed infections in setting of hardware placement after infection, but what about hardware associated infection when placed for primary orthopedic indication?
- As Laura discussed, some people may make a distinction as to whether the infection was “superficial” or below the fascia >> but it is difficult to reliably distinguish between the two, especially for early post-operative infections when the fascia not yet healed
- Treatment will depend on the depth of infection and whether or not all the hardware is removed
- An approach that is often used: IV therapy will be continued until bone fusion is achieved >> if removal of hardware is feasible, repeat cultures at the time of hardware removal can help guide subsequent therapy >> if hardware can’t be removed, consider suppression (more on that below)
- Removal of hardware may improve outcomes, especially for Staph, but it is not always practical
- Laura explained how she approaches these cases with shared decision making between the ID team, the patient, and their surgeon. There’s no test to tell us when a patient is cured or that every last bacteria has been eradicated – so we have to discuss what are the risks of relapse? What do you think about ongoing antibiotics? What surgery might you need in that case? What would that mean for your life? Those considerations can help guide your management
So your patient completes their antibiotic treatment course – should we use suppressive PO antibiotics when hardware remains in place? If so, how?
- We don’t really know yet
- There is not a lot of clinical data to guide treatment decisions in this population
- Here are a few resources considering role of suppressive antibiotics for spinal infections with hardware in place:
- Beydoun N, Tandon S, Krengel S, et al. A Retrospective Chart Review on the Role of Suppressive Therapy in the Management of Spinal Infections Involving Hardware. Open Forum Infect Dis. 2020;7(7):ofaa253. Published 2020 Jun 25. doi:10.1093/ofid/ofaa253
- Cho OH, Bae IG, Moon SM, et al. Therapeutic outcome of spinal implant infections caused by Staphylococcus aureus: A retrospective observational study. Medicine (Baltimore). 2018;97(40):e12629. doi:10.1097/MD.0000000000012629
- Lall RR, Wong AP, Lall RR, Lawton CD, Smith ZA, Dahdaleh NS. Evidence-based management of deep wound infection after spinal instrumentation. J Clin Neurosci. 2015;22(2):238-242. doi:10.1016/j.jocn.2014.07.010
Infographics
Goal
Listeners will be able to diagnose vertebral osteomyelitis
Learning Objectives
After listening to this episode, listeners will be able to:
- Identify risk factors for vertebral osteomyelitis
- Describe the clinical evaluation of vertebral osteomyelitis
- Discuss the potential duration of treatment for native vertebral osteomyelitis and hardware associated spinal infection
Disclosures
Our guest (Laura Certain) as well as Febrile podcast and hosts report no relevant financial disclosures
Citation
Certain, L., Niehaus, E., Dong, S. “#50: Bad to the Bone”. Febrile: A Cultured Podcast. https://player.captivate.fm/episode/25eda8f8-11be-4abb-a9f3-2acc8a01dfbc


1 thought on “Episode #50 – Bad to the Bone”
Thanks for the post! Do you know why vertebral TB osteomyelitis tend to spare the disc meanwhile pyogenic spondylodiscitis don’t?
Comments are closed.