Table of Contents
Credits
Host: Sara Dong
Guests: Jame McCrae, Callum Mutch
Producing/Editing/Cover Art: Sara Dong
Our Guests

Jame McCrae & Callum Mutch from the ID:IOTS Podcast!
ID:IOTS = Infectious Diseases: Insights of Two Specialists
Consult Notes
Basics & Background for Aminoglycosides
- Can be classified by source organism with -mycin or -micin
- -mycin (Streptomyces): streptomycin, kanamycin, neomycin, paromomycin
- -micin (Micromonospora): amikacin, gentamicin
- Groups by chemical structure:
- Streptomycin group: streptomycin
- Neomycin group: neomycin, paromomycin
- Kanamycin: gentamicin, tobramycin, amikacin, plazomicin
- Structure:
- Amine (protein bit) + glycoside (sugary bit)
- Highly polarised → stays where it’s put (topical, blood, urine)
- Mechanism of action:
- Inhibits protein synthesis by irreversibly binding to the bacterial 30S ribosome subunit → Misreading of mRNA codons → Bacteriostatic
- Drug is cationic → Binds anionic cell membrane components → Reduced membrane integrity → Cell lysis → Bactericidal
- History:
- Discovered in 1943 (streptomycin and neomycin) → 1st TB treatment
- Last aminoglycoside licensed: plazomicin in 2018
What is the spectrum of activity for aminoglycosides?
- Active against most Gram-negatives, including enteric GNRs, Pseudomonas, and Acinetobacter
- Poor activity against anaerobes, atypical respiratory tract pathogens, Gram positive organisms
- When used in combination with another medication, can exhibit activity against Gram positive organisms like Staph, Strep, and Enterococci
- As monotherapy, aminoglycosides don’t achieve appropriate intracellular concentrations for activity against Gram positive organisms
What are the typical clinical uses of aminoglycosides?
- Combination antibacterial therapy for serious Gram negative infections (such as pneumonia, sepsis), usually in combination with a beta-lactam
- This is in hopes that your bug is susceptible to at least one agent in the combination (then usually discontinued in favor of less toxic alternatives)
- Certain organisms like Listeria
- Directed therapy for resistant organisms (may be in combination with others for additive/synergistic activity)
- Combination for invasive enterococcal infections like endocarditis and sometimes certain Strep > but only if no high level AG resistance, can tolerate toxicity [combo for GP organisms for endocarditis, osteomyelitis, sepsis)
- Antimycobacterial (mostly amikacin)
- Monotherapy is typically less common, but here are some examples:
- Plague & tularemia: streptomycin, gentamicin
- UTIs with resistant organisms
- [not in US but spectinomycin as alternative for nonpharyngeal gonococcoal infection]
- Poor activity and/or penetration in lungs, abscesses, CNS >> so likely not going to use as monotherapy in those situations
- Paromomycin = E.histolytica
Some notes on dosing of aminoglycosides
- We didn’t focus on this as much in the episode, but here are a few notes/references
- Historically conventional AG dosing was 2 – 2.5 mg/kg IV Q8h (so 6 – 7.5 mg/kg/day) → BUT AGs have concentration dependent killing (i.e. Cmax/MIC is what matters)
- Jame mentioned this reference: Nicolau DP, Freeman CD, Belliveau PP, Nightingale CH, Ross JW, Quintiliani R. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother. 1995;39(3):650-655. doi:10.1128/AAC.39.3.650
- Trialled 7 mg/kg once daily and found better side effect profile (acute kidney injury 4% vs. 1.2%), same efficacy
- Gentamicin or tobramycin extended-interval dosing is typically 5-7 mg/kg once daily
- Trough goal: <1 μg/mL
- Extended interval dosing takes advantage of concentration-dependent killing and postantibiotic effect
- Multiple trials in adult and pediatric patients have shown comparable efficacy of extended interval dosing with the traditional dosing
- Munckhof WJ, Grayson ML, Turnidge JD. A meta-analysis of studies on the safety and efficacy of aminoglycosides given either once daily or as divided doses. J Antimicrob Chemother. 1996;37(4):645-663. doi:10.1093/jac/37.4.645
- Smyth AR, Bhatt J. Once-daily versus multiple-daily dosing with intravenous aminoglycosides for cystic fibrosis. Cochrane Database Syst Rev. 2012;(2):CD002009. Published 2012 Feb 15. doi:10.1002/14651858.CD002009.pub4
- Contopoulos-Ioannidis DG, Giotis ND, Baliatsa DV, Ioannidis JP. Extended-interval aminoglycoside administration for children: a meta-analysis. Pediatrics. 2004;114(1):e111-e118. doi:10.1542/peds.114.1.e111
- Mavros MN, Polyzos KA, Rafailidis PI, Falagas ME. Once versus multiple daily dosing of aminoglycosides for patients with febrile neutropenia: a systematic review and meta-analysis. J Antimicrob Chemother. 2011;66(2):251-259. doi:10.1093/jac/dkq451
- Rao SC, Ahmed M, Hagan R. One dose per day compared to multiple doses per day of gentamicin for treatment of suspected or proven sepsis in neonates. Cochrane Database Syst Rev. 2006;(1):CD005091. Published 2006 Jan 25. doi:10.1002/14651858.CD005091.pub2
- Gentamicin or tobramycin dosing for Gram-positive synergy is typically 1 mg/kg every 8-12 hrs
- Peak goal: 3-4 μg/mL with trough <1 μg/mL
Safety / Adverse effects of aminoglycosides
- Nephrotoxicity → dose-related
- Mechanism of renal failure: acute tubular necrosis
- More likely to occur with multiple daily dosing rather than once daily dosing (so consider extended interval dosing)
- Ensure adequate troughs and correct dosing weight
- Ototoxicity → usually irreversible
- May cause vestibular (vertigo, disequilibrium, nausea, vomiting) or cochlear (tinnitus, hearing loss) toxicity
- May even occur with optimal levels and once daily dosing
- Neuromuscular blockade → rare
- Higher risk in those with high doses and when given soon after general anesthesia or neuromuscular blockers
- May exacerbate muscle weakness with myasthenia gravis
- Drug interactions
- Use with caution in those on concomitant nephrotoxic medications
- Toxicity is usually dose-related! Make sure to ensure the right doses are used based on correct body weight, CrCl
- Obese patients should be dosed with adjusted body weight; Underweight patients should be dosed on total body weight
Side note: Drugs that may unmask or worsen myasthenia gravis
This occasionally is a board questions, but consider these antibiotics: aminoglycosides, fluoroquinolones, macrolides, chloroquine
Jame and Callum discussed the Scotland adoption of aminoglycoside based empirical antibiotic regimens
- They detailed some information from the Vale of Leven C.difficile outbreak. You can find more about the inquiry here. A quick summary of some facts
- From Jan 2007 to December 2008, 143 patients tested positive for C.difficile infection
- The total number of deaths attributed to CDI was 34, which could be an underestimate
- The inquiry reported multiple failings and made 75 recommendations, which included recommendations related to infection prevention and control, nursing and medical care, antibiotic prescribing, communication with patients and relatives, and death certification. Final report available here from National Records of Scotland
- Starting in 2008 (Lanarkshire) and ending in 2014 (Lothian), the NHS Scotland moved from a broad-spectrum to narrow-spectrum empiric antibiotic regimen
- As Jame explained in his example, say you want to cover someone for ‘sepsis, unknown source’. Your options are:
Strep | Staph aureus | Gram negatives | Anaerobes |
Amoxicillin | Gentamicin | Metronidazole | |
Ceftriaxone / Ceftazidime / Cefepime | Metronidazole | ||
Amoxicillin-clavu (Augmentin/Co-amoxiclav) / Piperacillin-Tazobactam / Meropenem | |||
- Scotland does option 1. The effects/goals of this option:
- Less C.difficile
- Lower MRSA rates
- Lower mortality from Gram negative sepsis
- No difference in ICU admission, length of stay, need for RRT
- No increase in AG resistance
- Lower co-amoxiclav/ceftriaxone resistance
- Check out these references:
- Lawes T, Lopez-Lozano JM, Nebot CA, et al. Effects of national antibiotic stewardship and infection control strategies on hospital-associated and community-associated meticillin-resistant Staphylococcus aureus infections across a region of Scotland: a non-linear time-series study [published correction appears in Lancet Infect Dis. 2016 Jan;16(1):16]. Lancet Infect Dis. 2015;15(12):1438-1449. doi:10.1016/S1473-3099(15)00315-1
- Ritchie ND, Irvine SC, Helps A, Robb F, Jones BL, Seaton RA. Restrictive antibiotic stewardship associated with reduced hospital mortality in gram-negative infection. QJM. 2017;110(3):155-161. doi:10.1093/qjmed/hcw134
So why doesn’t everyone do this?
- This approach has been used previously
- It is easier to give ceftriaxone+metronidazole or co-amox: no need to need drug monitoring with trough levels
- Most places do not have unified approach to prescribing AG (such as teaching medical and nursing staff, automatic calculators for dosage, checking with pharmacy, audit of practice)
- EUCAST also provided significant guidance against use of AGs in general, particularly with gentamicin for Pseudomonas
- Here are the breakpoint comparisons mentioned on the show:
Bug | CLSI | EUCAST | ||||
S | I | R | S | I | R | |
Enterobacterales | ≤4 | 8 | 16+ | ≤2 | >2 | |
Pseudomonas | ≤4 | 8 | 16+ | ≤2 (Tobra) | >2 (Tobra) | |
- For gentamicin, EUCAST no longer recommended for Pseudomonas. You can find the Guidance Document on implementation and use of revised aminoglycoside breakpoints from April 2020 here
- Looked at older literature (variable dosing, multiple times a day) and concluded not safe for systemic use, certainly as monotherapy
- Acceptable for use in urinary tract due to good levels in urine/renal tissue
- Callum discussed Pseudomonas ECOFF, which is ~8 for gentamicin – compared to 2 for tobra / 16 for amikacin
- (Epidemiologic cutoff values, abbreviated ECV (CLSI) or ECOFF (Eucast) , are measures of a drug MIC distribution that separate bacterial populations into those representative of a wild type population, and those with acquired or mutational resistance to the drug)
- Scotland’s response to this EUCAST statement is summarized in this SBAR
- Extensive experience of Gentamicin 5-7mg/kg 24-hourly as the gram-negative ‘backbone’ of empirical therapy
- This was not utilized when EUCAST did their literature search
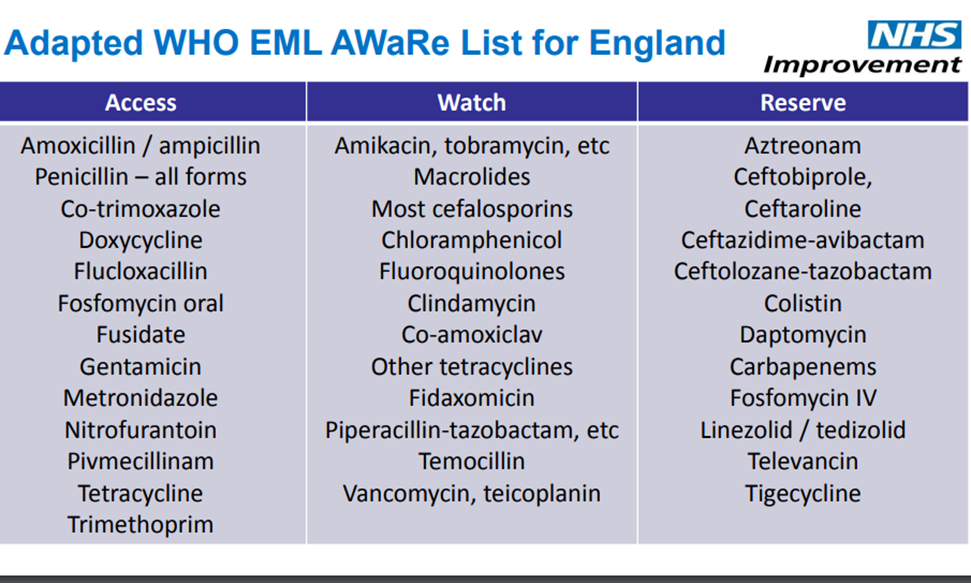
Jame also provided an explanation of the Access, Watch, and Reserve categories for antibiotics
- The WHO Model Lists of Essential Medicines are updated about every two years, and the WHO AWaRe antibiotic categorization classifies antibiotics into three groups based on potential to induce and propagate resistance
- Access: 1st or 2nd choice antibiotics, offer best therapeutic value while minimizing potential for resistance
- Watch: 1st or 2nd choice antibiotics, only indicated for specific limited number of infective syndromes, more prone to be a target of antibiotic resistance (thus prioritized as targets of stewardship programs and monitoring)
- Reserve: last resort antibiotics for highly selected patients (life-threatening infections due to multi-drug resistant bacteria), closely monitored and prioritized as targets of stewardship programs to ensure their continued effectiveness
- Not all antibiotics are included in the AWaRe framework, but you can look through the list of antibiotics here
- Jame also provided an adapted list for England, which differs slightly to fit practice
Other resources and links, including the -static/-cidal debate and C.diff:
- You can check out this Antimicrobial Companion website to see some of the educational materials available from NHS Lothian, especially on gentamicin
- We briefly touched on the bacteriostatic vs bactericidal debate. You can read more here about this myth:
- Wald-Dickler N, Holtom P, Spellberg B. Busting the Myth of “Static vs Cidal”: A Systemic Literature Review. Clin Infect Dis. 2018;66(9):1470-1474. doi:10.1093/cid/cix1127
- Here is a webinar from 2018 from the authors for CIDRAPASP
- G. A. Pankey, L. D. Sabath, Clinical Relevance of Bacteriostatic versus Bactericidal Mechanisms of Action in the Treatment of Gram-Positive Bacterial Infections, Clinical Infectious Diseases, Volume 38, Issue 6, 15 March 2004, Pages 864–870, https://doi.org/10.1086/381972
- Wald-Dickler N, Holtom P, Spellberg B. Busting the Myth of “Static vs Cidal”: A Systemic Literature Review. Clin Infect Dis. 2018;66(9):1470-1474. doi:10.1093/cid/cix1127
- Here are two resources for thinking about antibiotics and the risk of C.diff infection below. Antibiotics that are frequently implicated include fluoroquinolones, clindamycin, and broad-spectrum penicillins and cephalosporins – but any antibiotics could predispose to colonization by C.diff
- Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother. 2013;57(5):2326-2332. doi:10.1128/AAC.02176-12
- Deshpande A, Pasupuleti V, Thota P, et al. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother. 2013;68(9):1951-1961. doi:10.1093/jac/dkt129
- Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis. 2011;53(1):42-48. doi:10.1093/cid/cir301
Infographics
Disclosures
Our guests (Jame McCrae and Callum Mutch) as well as Febrile podcast and hosts report no relevant financial disclosures
Citation
McCrae, J., Mutch, C., Dong, S. “#51: Febrile Digest – Across the Pond with ID:IOTS podcast ”. Febrile: A Cultured Podcast. https://player.captivate.fm/episode/0777091c-0991-42d4-89a9-4032d77b4fa5


