Table of Contents
Credits
Host: Sara Dong
Guests: Jillian Hayes, Erin McCreary
Writing/Producing/Editing/Cover Art: Sara Dong
Infographics: Sara Dong
Our Guests
Jillian Hayes, PharmD, BCIDP

Dr. Hayes is an infectious diseases and antimicrobial stewardship clinical pharmacist at Duke University Hospital in Durham, NC. She obtained her Doctor of Pharmacy degree from the greatest university in the world, the University of South Carolina (go gamecocks), and completed her residency at Vanderbilt University Medical Center. She is an active member of the Society of Infectious Diseases Pharmacists, currently serving as the vice chair of the Publications and Podcasts Committee. Her interests within infectious diseases include antimicrobial stewardship in transitions of care, incorporation of trainees into antimicrobial stewardship, and resident wellness and mentoring.
Erin McCreary, PharmD, BCPS, BCIDP

Dr. McCreary is an infectious diseases pharmacist serving as the Director of Infectious Diseases Improvement and Clinical Research Innovation for UPMC health system and a Clinical Assistant Professor at the University of Pittsburgh School of Medicine. She obtained her Doctor of Pharmacy degree from the actual greatest university in the world, Auburn University (war eagle), and completed residency at the University of Wisconsin. She hosts Breakpoints, the SIDP podcast and serves on the SIDP Executive Board. Her research focuses on infectious diseases in immunocompromised hosts, gram-negative resistance, and antimicrobial stewardship implementation science.
Culture
Jillian mentioned the nostalgic joy/excitement from Disney Channel crossovers of the past! In addition, she loves to dance and hopes to one day be on Dancing with the Stars 🙂
Erin is a proud mother of an 18 month old golden retriever named Nora. She also mentioned being born in Thailand and how her family culture/art is often Thai despite the fact that she is Icelandic. Lastly, she loves being on the water.
Consult Notes
Case Summary
This case was a bit of “make your own adventure” to discuss various points related to antifungals, but the initial patient stem was a 50 year old with renal transplant who presented with fever, cough, and chest pain
Key Points
Let’s start with a quick note on empiric antifungal therapy
- In absence of bloodstream infection or clear evidence of invasive candidiasis, the decision to use empiric antifungal therapy will depend on individual circumstances (such as patients with neutropenic fever who might receive empiric therapy due to risk of candidiasis)
- For non-neutropenic patients with sepsis, routine antifungal therapy in initial regimen is usually not warranted
- For those non-neutropenic patients who are critically ill with persistent fever or hypotension despite broad-spectrum antibiotics, the benefit of empiric antifungal therapy is uncertain and there are not clear clinical criteria to help guide this decision
- The 2016 IDSA guidelines for management of candidiasis recommend empiric antifungal therapy for critically ill patients with persistent unexplained fever and risk factors for invasive candidiasis
- Risk factors: indwelling central venous catheter, parenteral nutrition, hemodialysis, trauma or burns, recent surgery (especially abdominal surgery), cultures demonstrating Candida colonization at nonsterile sites
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1-e50. doi:10.1093/cid/civ933
- Generally this empiric agent will be an echinocandin although fluconazole might be an option in certain patients who have not received prior azole therapy
- There is not a clear benefit in the available clinical studies using empiric antifungals in non-neutropenic patients with persistent signs of infection although some of the earlier studies in patients who had bowel surgery/perforation suggested possible benefit of empiric fluconazole
- EMPIRICUS RCT: Timsit JF, Azoulay E, Schwebel C, et al. Empirical Micafungin Treatment and Survival Without Invasive Fungal Infection in Adults With ICU-Acquired Sepsis, Candida Colonization, and Multiple Organ Failure: The EMPIRICUS Randomized Clinical Trial. JAMA. 2016;316(15):1555-1564. doi:10.1001/jama.2016.14655
- Knitsch W, Vincent JL, Utzolino S, et al. A randomized, placebo-controlled trial of preemptive antifungal therapy for the prevention of invasive candidiasis following gastrointestinal surgery for intra-abdominal infections. Clin Infect Dis. 2015;61(11):1671-1678. doi:10.1093/cid/civ707
- Piarroux R, Grenouillet F, Balvay P, et al. Assessment of preemptive treatment to prevent severe candidiasis in critically ill surgical patients. Crit Care Med. 2004;32(12):2443-2449. doi:10.1097/01.ccm.0000147726.62304.7f
- Shan YS, Sy ED, Wang ST, Lee JC, Lin PW. Early presumptive therapy with fluconazole for occult Candida infection after gastrointestinal surgery. World J Surg. 2006;30(1):119-126. doi:10.1007/s00268-005-7807-z
We discussed what factors impact micafungin dosing, especially obesity
- In general, Erin emphasized that echinocandins are safe and well tolerated!!
- Echinocandins demonstrate drug exposure – efficacy relationships, and the maximum concentration/minimal inhibitory concentration ratio (Cmax/MIC) and area under the concentration-time curve/MIC ratio (AUC/MIC) are proposed PK/PD markers for clinical response
- Micafungin may require dosing adjustments based on:
- MIC of specific Candida spp
- Weight of patient
- Age (but we aren’t addressing pediatric dosing in this episode since its an adult case)
- Critical illness (hypoalbuminemia decreases AUC by 30%)
- No adjustment required with hepatic or renal impairment
- We focused on obesity and critical illness during this episode
- Obesity
- PK studies and some clinical data suggest suboptimal exposures of echinocandins with obesity
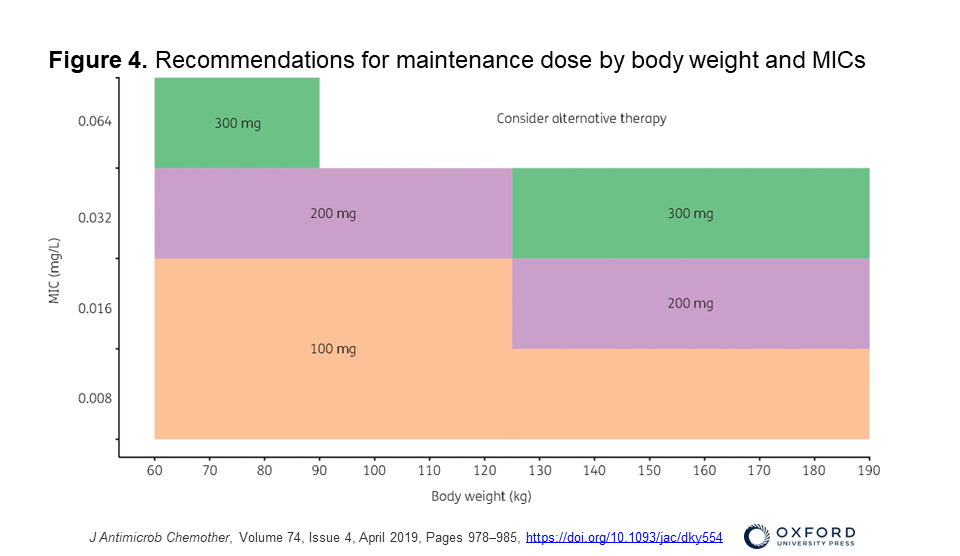
- This 2019 paper was a prospective PK study mentioned by Erin: Wasmann RE, Smit C, Ter Heine R, et al. Pharmacokinetics and probability of target attainment for micafungin in normal-weight and morbidly obese adults. J Antimicrob Chemother. 2019;74(4):978-985. doi:10.1093/jac/dky554
- Check out Figure 4 for recommendations for maintenance dose by body weight and MICs (available in color in the online version)
- A 100mg dose is appropriate if weigh <125kg and MIC 0.016 for Candida — if >125kg weight, a dose of 200mg would be needed for MIC of 0.016 or higher
- Models are important, but does that pan out clinically? There is some clinical data Erin mentioned:
- No differences in mortality among patients with candidemia across BMI category (with anidulafungin) in this 2022 paper: Hutton M, Kenney RM, Vazquez JA, Davis SL. Influence of Body Weight Category on Outcomes in Candidemia Patients Treated With Anidulafungin. J Pharm Pract. 2022;35(1):20-25. doi:10.1177/0897190020938219
- Is it safe to push the dose of echinocandins? Yes, up to 900mg/d safely
- Grant VC, Nguyen K, Rodriguez S, Zhou AY, Abdul-Mutakabbir JC, Tan KK. Characterizing Safety and Clinical Outcomes Associated with High-Dose Micafungin Utilization in Patients with Proven Invasive Candidiasis. Trop Med Infect Dis. 2022;7(2):23. Published 2022 Feb 3. doi:10.3390/tropicalmed7020023
- This study of 21 adult patients who received 200mg or more of micafungin for at least 3 days for proven invasive candidiasis from 2013-2021 (median BMI 37)
- Average dose 300mg
- AST/ALT ok, some increase in alk phos
- 4 patients died (17.4%)
- No comparator but basically no safety signals
- Grant VC, Nguyen K, Rodriguez S, Zhou AY, Abdul-Mutakabbir JC, Tan KK. Characterizing Safety and Clinical Outcomes Associated with High-Dose Micafungin Utilization in Patients with Proven Invasive Candidiasis. Trop Med Infect Dis. 2022;7(2):23. Published 2022 Feb 3. doi:10.3390/tropicalmed7020023
- Models are important, but does that pan out clinically? There is some clinical data Erin mentioned:
- Critical illness
- Boonstra JM, van der Elst KC, Veringa A, et al. Pharmacokinetic Properties of Micafungin in Critically Ill Patients Diagnosed with Invasive Candidiasis. Antimicrob Agents Chemother. 2017;61(12):e01398-17. Published 2017 Nov 22. doi:10.1128/AAC.01398-17
- Exposures are lower in critically ill patients and those weighing >100kg
- Critical illness effects antibacterials similarly: increased clearance, increased volume, deranged PK
- Higher MIC, less likely to hit your target
- Anti-infective Drugs Committee of the International Association of Therapeutic Drug Monitoring (TDM) and Clinical Toxicity published a position statement in 2022 suggesting echinocandin TDM in patients at known risk of suboptimal drug exposure
- Kim HY, Baldelli S, Märtson AG, et al. Therapeutic Drug Monitoring of the Echinocandin Antifungal Agents: Is There a Role in Clinical Practice? A Position Statement of the Anti-Infective Drugs Committee of the International Association of Therapeutic Drug Monitoring and Clinical Toxicology. Ther Drug Monit. 2022;44(1):198-214. doi:10.1097/FTD.0000000000000931
- TDM may be valuable in patients with critical illness, obesity, moderate liver impairment, drug-drug interactions, hypoalbuminemia, and those undergoing extracorporeal membrane oxygenations (as these are associated with altered exposure to caspofungin and/or micafungin)
- That said, most people/institutions likely don’t have easy access to TDM for echinocandins
- Boonstra JM, van der Elst KC, Veringa A, et al. Pharmacokinetic Properties of Micafungin in Critically Ill Patients Diagnosed with Invasive Candidiasis. Antimicrob Agents Chemother. 2017;61(12):e01398-17. Published 2017 Nov 22. doi:10.1128/AAC.01398-17
- Patients with cancer may have higher clearance as well: Alqahtani S, Alfarhan A, Alsultan A, Alsarhani E, Alsubaie A, Asiri Y. Assessment of Micafungin Dosage Regimens in Patients with Cancer Using Pharmacokinetic/Pharmacodynamic Modeling and Monte Carlo Simulation. Antibiotics (Basel). 2021;10(11):1363. Published 2021 Nov 8. doi:10.3390/antibiotics10111363
- Some additional resources/references:
- Barber KE, Wagner JL, Miller JM, Lewis EA, Stover KR. Impact of Obesity in Patients with Candida Bloodstream Infections: A Retrospective Cohort Study. Infect Dis Ther. 2020;9(1):175-183. doi:10.1007/s40121-020-00285-7
- Vossen MG, Knafl D, Haidinger M, et al. Micafungin Plasma Levels Are Not Affected by Continuous Renal Replacement Therapy: Experience in Critically Ill Patients. Antimicrob Agents Chemother. 2017;61(8):e02425-16. Published 2017 Jul 25. doi:10.1128/AAC.02425-16
- Andes D, Ambrose PG, Hammel JP, et al. Use of pharmacokinetic-pharmacodynamic analyses to optimize therapy with the systemic antifungal micafungin for invasive candidiasis or candidemia. Antimicrob Agents Chemother. 2011;55(5):2113-2121. doi:10.1128/AAC.01430-10
- Pappas PG, Rotstein CM, Betts RF, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis [published correction appears in Clin Infect Dis. 2008 Jul 15;47(2):302]. Clin Infect Dis. 2007;45(7):883-893. doi:10.1086/520980
Next up we discussed antifungal therapy in setting of ECMO. Here are some of the considerations that Jillian explained
- Drugs can get sequestered in the ECMO circuit itself: they bind to the tubing, the oxygenator, which leads to decreased serum concentrations
- Drugs with higher protein binding often see more sequestration
- Volume of distribution is important
- The circuit provides a large surface area / volume of distrbution
- These patients are typically getting a ton of fluid resuscitation (which even independent of ECMO, will increase volume of distribution)
- Endothelial activation and leaky capillaries also increase the volume of distribution
- Higher lipophilicity = ↑extraction from circuit
- In addition, these patients often will have hepatic dysfunction or require renal replacement therapy, which would also affect drug exposure
Erin briefly spoke about specific antifungal therapy while on ECMO
- Micafungin in ECMO:
- Conflicting data: some ex vivo studies suggest high extraction while others say no clinically significant effect on PK
- Typically we won’t change the dose with ECMO alone … but the patients are usually critically ill and/or obese, so there may be other reasons to adjust the dose
- Amphotericin & ECMO:
- Liposomal amphotericin daily dosing is the preferred ampho product in most centers, but some favor use of deoxycholate in the setting of ECMO. More about that below
- The first publication with TDM showing low exposure: This had a case of blastomycosis requiring mechanical ventilation, ECMO, CRRT where the authors describe concern that liposomal Amphotericin B was significantly removed by ECMO (getting sequestered)
- This publication suggested 2-fold increase in standard total daily dose needed for liposomal amphotericin
- Some real world evidence of people switching
Some additional resources/references:
- Roberts JA, Bellomo R, Cotta MO, et al. Machines that help machines to help patients: optimising antimicrobial dosing in patients receiving extracorporeal membrane oxygenation and renal replacement therapy using dosing software. Intensive Care Med. 2022;48(10):1338-1351. doi:10.1007/s00134-022-06847-2
- Honore PM, De Bels D, Gutierrez LB, et al. Optimizing micafungin dosing in critically ill patients: what about extracorporeal therapies?. Crit Care. 2018;22(1):289. Published 2018 Nov 1. doi:10.1186/s13054-018-2231-6
- Shah A, Sampathkumar P, Stevens RW, et al. Reducing Broad-Spectrum Antimicrobial Use in Extracorporeal Membrane Oxygenation: Reduce AMMO Study. Clin Infect Dis. 2021;73(4):e988-e996. doi:10.1093/cid/ciab118
- Donadello K, Antonucci E, Cristallini S, et al. β-Lactam pharmacokinetics during extracorporeal membrane oxygenation therapy: A case-control study. Int J Antimicrob Agents. 2015;45(3):278-282. doi:10.1016/j.ijantimicag.2014.11.005
- Shekar K, Roberts JA, Mcdonald CI, et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care. 2012;16(5):R194. Published 2012 Oct 15. doi:10.1186/cc11679
- Wildschut ED, Ahsman MJ, Allegaert K, Mathot RA, Tibboel D. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. 2010;36(12):2109-2116. doi:10.1007/s00134-010-2041-z
- Zhao Y, Seelhammer TG, Barreto EF, Wilson JW. Altered Pharmacokinetics and Dosing of Liposomal Amphotericin B and Isavuconazole during Extracorporeal Membrane Oxygenation. Pharmacotherapy. 2020;40(1):89-95. doi:10.1002/phar.2348
- Watt KM, Cohen-Wolkowiez M, Williams DC, et al. Antifungal Extraction by the Extracorporeal Membrane Oxygenation Circuit. J Extra Corpor Technol. 2017;49(3):150-159.
Here is a ASHP Drug Shortage graphic
AZOLE TIME! There will be a summary from Erin’s masterclass on azoles in the graphics! In addition, here are some other references
Here are some useful papers on azole TDM that Erin mentioned during the show:
- Chau MM, Daveson K, Alffenaar JC, et al. Consensus guidelines for optimising antifungal drug delivery and monitoring to avoid toxicity and improve outcomes in patients with haematological malignancy and haemopoietic stem cell transplant recipients, 2021. Intern Med J. 2021;51 Suppl 7:37-66. doi:10.1111/imj.15587
- Ashok A, Mangalore RP, Morrissey CO. Azole Therapeutic Drug Monitoring and its Use in the Management of Invasive Fungal Disease. Current Fungal Infection Reports. 2022;16:55-69. DOI: 10.1007/s12281-022-00430-4
- Kably B, Launay M, Derobertmasure A, Lefeuvre S, Dannaoui E, Billaud EM. Antifungal Drugs TDM: Trends and Update. Ther Drug Monit. 2022;44(1):166-197. doi:10.1097/FTD.0000000000000952
- A summary of these created by Erin McCreary is below:
Agent | Ashok et al. (10.1007/s12281-022-00430-4) | Chau et al. (10.1111/imj.15587) | Kably et al. (10.1097/FTD.0000000000000952)
|
Itraconazole | Treatment: Routine for treatment irrespective of formulation Prophylaxis: Capsules – Routine for prophylaxis Solution – Routine for prophylaxis SUBA-Itra – Selected cases at risk of low exposure (e.g. DDIs) Other: TDM for patients who initiate/discontinue interacting med, undergo a formulation change, or demonstrate lack of response or sign of toxicity Ranges: Treatment – >1-1.5 mg/L Prophylaxis – >0.25-0.5 mg/L Toxicity – 3-5 mg/L Timing: 5-7 days (loading dose); troughs 10-14 days (no loading dose); troughs Thereafter, every 1-2 weeks once steady state achieved | Indication: To ensure adequate absorption; therapeutic concentration
Treatment: Routine for treatment irrespective of formulations Prophylaxis: Capsules – Routeine for prophylaxis Solution – Routine for prophylaxis SUBA-Itra – Recommended in selected cases at risk of low exposure (e.g. DDIs, GI complications, and young children) Ranges: Treatment – 1-4 mg/L (HPLC) (AII for efficacy, BIII for toxicity) Prophylaxis – 0.5-4 mg/L (HPLC) (AII for efficacy, BIII for toxicity) Timing: 5-7 days (loading dose); troughs 10-14 days (no loading dose); troughs Adjustment: – If subtx, increase dose by 25-50% – If taking capsules, also consider switch to solution or SUBA-Itra – Ensure capsule taken with food, avoid H2RA/PPIs – Ensure solution on empty stomach | “ITZ TDM is still recommended, but the benefit-to-risk ratio for prolonged prophylaxis in CF is a matter of debate.”
TDM Recommendation: >0.5-1 mg/L (various references) |
Voriconazole | Treatment: Routine for treatment irrespective of formulation Prophylaxis: Limited evidence for routine TDM in prophylaxis Ranges: Treatment – >1.7-2 mg/L; >2 mg/L if high risk, poor prognosis, bulky disease Prophylaxis – not established Toxicity – 4-6 mg/L; routine LFT monitoring and for clinical signs of neurotoxicity recommended Timing: Within 2-5 days of initiating therapy Repeat at 3-5 days may be warranted in: Critical illness, suspected treatment failure, DDIs, or change in dosing | Indication: To detect therapeutic and toxic concentrations
Treatment: Routine for treatment Prophylaxis: Recommended for prophylaxis Ranges: Treatment – 1-5.5 mg/L (AII); CNS infection, bulky disease, multifocal infection > 2 mg/L (BIII) Prophylaxis – 1-5.5 mg/L (AII) Timing: 2-5 days; trough Adjustments: – 0.0–0.49 mg/L: increase by 50% – 0.5–0.99 mg/L: increase by 25% – 1.0–5.5 mg/L: no change – > 5.5 mg/L and asymptomatic: decrease dose by 25% – > 5.5 mg/L with drug-related toxicities: hold one dose and decrease subsequent doses by 50% | “VRZ is the most challenging. There is a large consensus that the drug must be detectable with a trough level .1 mg/L, but contradictory findings have been reported concerning the upper range for trough level. In patients with normal hepatic function, outside the loading dose period, in the absence of significant metabolic inhibition, trough levels .4 mg/L (the peak value established after the PK exploitation of pivotal trials) is unlikely to be achieved with the standard 200 mg ·2 dose. Moreover, safety issues limit the maintenance of high exposures and doses in clinical practice.367–369 The 1-5.5-6 mg/L trough target recommended on the basis of weak evidence is frequently applied as a 1–4 mg/L target, resulting, in clinical practice, in a median or average trough exposure of 1–3 mg/L.”
TDM Recommendation 1-2 to 4-6 mg/L (various references) |
Posaconazole | Treatment: Routine for treatment (not required for intravenous) Prophylaxis: Suspension– Routine for prophylaxis Tablets – Selected cases at risk for low exposure Intravenous – Not required Ranges: Treatment – >1-1.25 mg/L Prophylaxis – >0.5 mg/L Toxicity – > 4 mg/L associated with PIPH Timing: 7 days | Indication: To ensure adequate absorption; therapeutic conecntration
Treatment: Routine for treatment irrespective of formulations Prophylaxis: Suspension – Routine for prophylaxis Tablets – Recommended for selected cases at risk of low exposure (e.g. DDIs, GI complications, young children) Ranges: Treatment – > 1 mg/L (AII) Prophylaxis – > 0.5 (AII for susp.; BII for tabs) Timing: After 5-7 days; troughs preferred, untimed concentrations usable given steady serum concentration over time Early monitoring (e.g. day 2) may be predictive of steady-state concentration Adjustments: Suspension: Prophylaxis: if subtherapeutic, increase to 200 mg four times daily or 300 mg three times daily Treatment: if subtherapeutic, increase to 400 mg three times daily Ensure patient taking suspension with food and/or acidic beverage, and avoid H2 antagonists and proton pump inhibitors Switch to modified-release formulation if patient can swallow tablets Tablets: If subtherapeutic, increase to 400 mg daily Consider administering with high fat meal if previously taken tablets in fasted state Intravenous: No data | “PSZ TDM is recommended, especially in curative situations. However, even recent recommendations refer to initial exposure achieved with the oral suspension and do not consider the new tablet formulation. In cases of prophylaxis, the low rate of events limits the demonstration of a clinically relevant threshold. The initial 0.5 or 0.7 mg/L threshold seems appropriate and the question as to whether less than 300 mg can be used with tablets is justified.”
TDM Recommendation > 1 mg/L (various references) |
Fluconazole | Treatment: Consider in select circumstances for IFD treatment: – Critical illness – Renal replacement – CNS infection – Treatment failure Ranges: Efficacy – Trough of 10-15 mg/L (or AUC/MIC > 50). However, not well elucidated. Toxicity – Threshold not well established. Timing: Optimal timing unclear | “We recommend against routine TDM of fluconazole (Not recommended, Level II evidence).”
Table: Timing/Ranges: None specified
Considerations: May be utilised in certain clinical circumstances for IFD treatment (e.g. critically ill patients with sepsis, patients with altered renal function, sanctuary site infections such as CNS, treatment failure or concerns for medication noncompliance) | “There is no major new insight regarding TDM for 5FC, FCZ, AmB, and echinocandins. […] The usual targets for FCZ are consistent over time, but the low level of recommendation for TDM raises a number of issues, particularly in cases of IV administration, impaired renal function, and ICU settings.” TDM Recommendation: 10-15 mg/mL (PMID: 32026399, 32535150) |
Isavuconazole | Treatment: Consider in select circumstances for IFD treatment: – Critical illness – Renal replacement – CNS infection – Treatment failure – GVHD – ECMO – Obesity Ranges: Efficacy – Not well elucidated. Toxicity – 5 mg/L, not consistently demonstrated. Timing: Single trough once steady state has been achieved (2-3 weks); value of repeat testing unclear | “No significant relationship was identified between drug exposure and mortality, clinical responses, overall response or safety outcomes in the SECURE study. However, interindividual variation exists and one trial has demonstrated exposure-related gastrointestinal side-effects, with increased incidence for steady-state concentrations between 4.87 and 5.13 mg/L.” mrda Table: “No” | “ISZ TDM is not generally recommended. However, ISZ use is frequently based on particular clinical features identified as beneficial by TDM.”
TDM Recommendation: > 1 mg/L (PMID: 32535150) |
Color Legend: Ashok et al. “Table 1. Azoles with most evidence for TDM”; Ashok et al. “Table 2. Azoles with emerging evidence for therapeutic drug monitoring”;
Some additional resources that I like as well
Check out table 1 for a quick review of spectrum of activity for systemic antifungal agents: Nett JE, Andes DR. Antifungal Agents: Spectrum of Activity, Pharmacology, and Clinical Indications. Infect Dis Clin North Am. 2016;30(1):51-83. doi:10.1016/j.idc.2015.10.012
I would draw your attention to Figure 7 with concentrations of various antifungals in tissue and body fluids, a great reference!! Felton T, Troke PF, Hope WW. Tissue penetration of antifungal agents. Clin Microbiol Rev. 2014;27(1):68-88. doi:10.1128/CMR.00046-13
Infographics
Goal
Listeners will be able to understand the adjustment of antifungal agents doses in clinical scenarios such as obesity and critical illness
Learning Objectives
After listening to this episode, listeners will be able to:
- Describe how micafungin dosing is impacted in obesity and critical illness
- Discuss use of amphotericin in setting of extracorporeal membrane oxygenation
- Compare and contrast azole antifungal agents in spectrum of activity and adverse effects
Disclosures
Our guests (Jillian Hayes, Erin McCreary) as well as Febrile podcast and hosts report no relevant financial disclosures
Citation
Hayes, J., McCreary, E., Dong, S. “#57 – Fun-Gals Just Want to have Fun: Antifungals with SIDP Breakpoints”. Febrile: A Cultured Podcast. https://player.captivate.fm/episode/bae6e35c-b909-493a-b9e5-6a909b1c5b92