Table of Contents
Credits
Host(s): Ralph Tayyar, Sara Dong
Guest: Jessica Ferguson
Writing: Ralph Tayyar, Jessica Ferguson, Sara Dong
Producing/Editing/Cover Art/Infographics: Sara Dong
Our Guests
Guest Co-Host
Ralph Tayyar, MD

Dr. Ralph Tayyar is currently a transplant or immunocompromised ID fellow at Stanford in Palo Alto CA, where he also completed his ID fellowship.
Guest Discussant
Jessica Ferguson, MD

Dr. Jessica Ferguson is a Clinical Assistant Professor in the Division of Infectious Diseases at Stanford University School of Medicine in Palo Alto, CA. She completed a one-year Transplant Infectious Disease fellowship at Stanford University in 2021 and joined the Stanford clinical faculty in August 2021. Her academic interests include medical education, transplant protocol development, CMV, antimicrobial resistance, and antibiotic stewardship.
Culture
Ralph was watching the World Cup games at the time of the recording, and Jessica was getting caught up on the TV show Billions
Consult Notes
Consult Q
What is the differential and diagnostic work-up for pulmonary nodules in an immunocompromised patient?
Case Summary
77 year old female with myelodysplastic syndrome transformed to AML who developed cough, neutropenic fever, and new pulmonary nodules – found to have invasive pulmonary aspergillosis due to A.fumigatus
Key Points
Risk factors for invasive fungal infections
- Prolonged neutropenia
- High dose glucocorticoids
- In HCT patients, specific risk factors include prolonged and profound neutropenia, glucocorticoids, unrelated or mismatched allogeneic HCT, and graft vs host disease
- Lung transplant – history of fungal infection or known colonization
- Viral infections – influenza, SARS-CoV-2, respiratory syncytial virus
- Resources:
Jessica discussed the differential for pulmonary nodules in immunocompromised patients, here is a brief summary of some of the typical causes:
- Infectious
- Fungal – Aspergillus, Mucormycoses, Scedosporium, Lomentosporium, Cryptococcus, Dimorphic fungi, nodular form of Pneumocystis jiroveci (rare) and others
- Mycobacterial – M. tuberculosis and nontuberculous mycobacteria
- Bacterial – bacterial abscess, septic emboli
- Parasitic – Paragonimiasis
- Viral – less likely
- Inflammatory
- Sarcoidosis
- Granulomatosis with polyangiitis
- Cryptogenic organizing pneumonia
- Malignancy – primary malignancy, metastases
Check out this paper and the figures (Fig 1 & 2 noted below): Azoulay E, Russell L, Van de Louw A, et al. Diagnosis of severe respiratory infections in immunocompromised patients. Intensive Care Med. 2020;46(2):298-314. doi:10.1007/s00134-019-05906-5
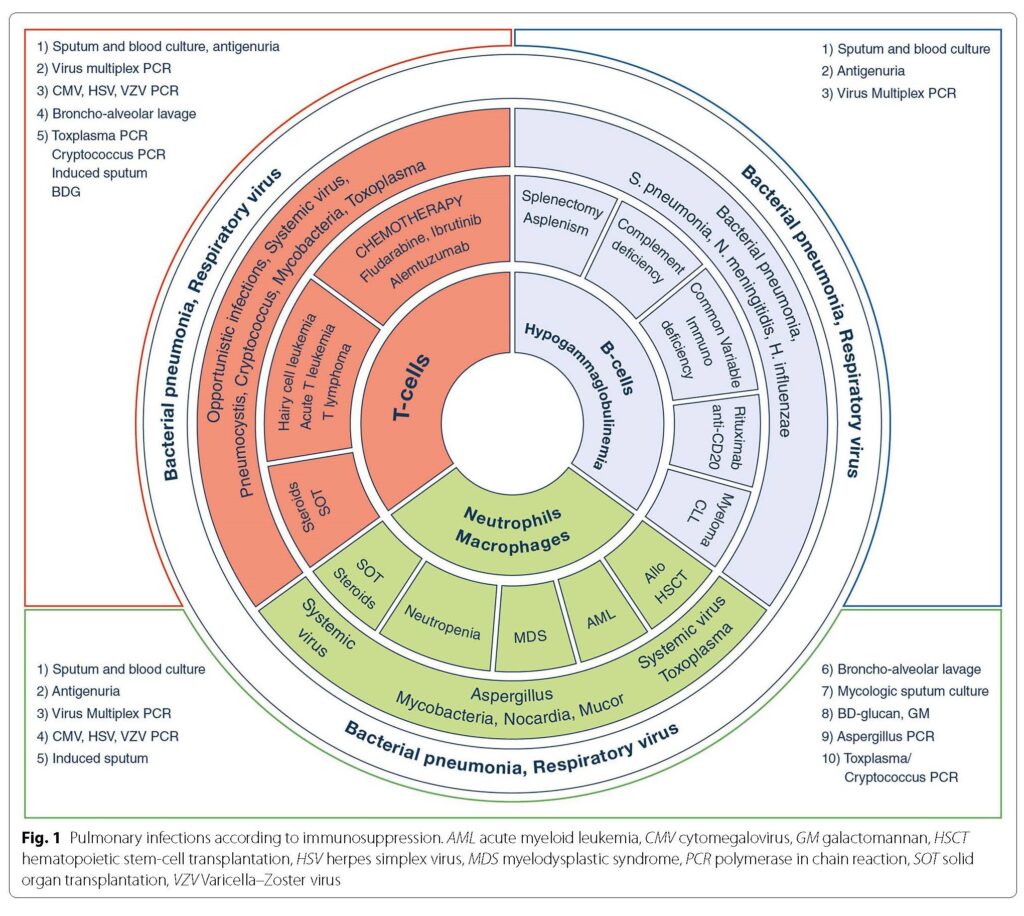
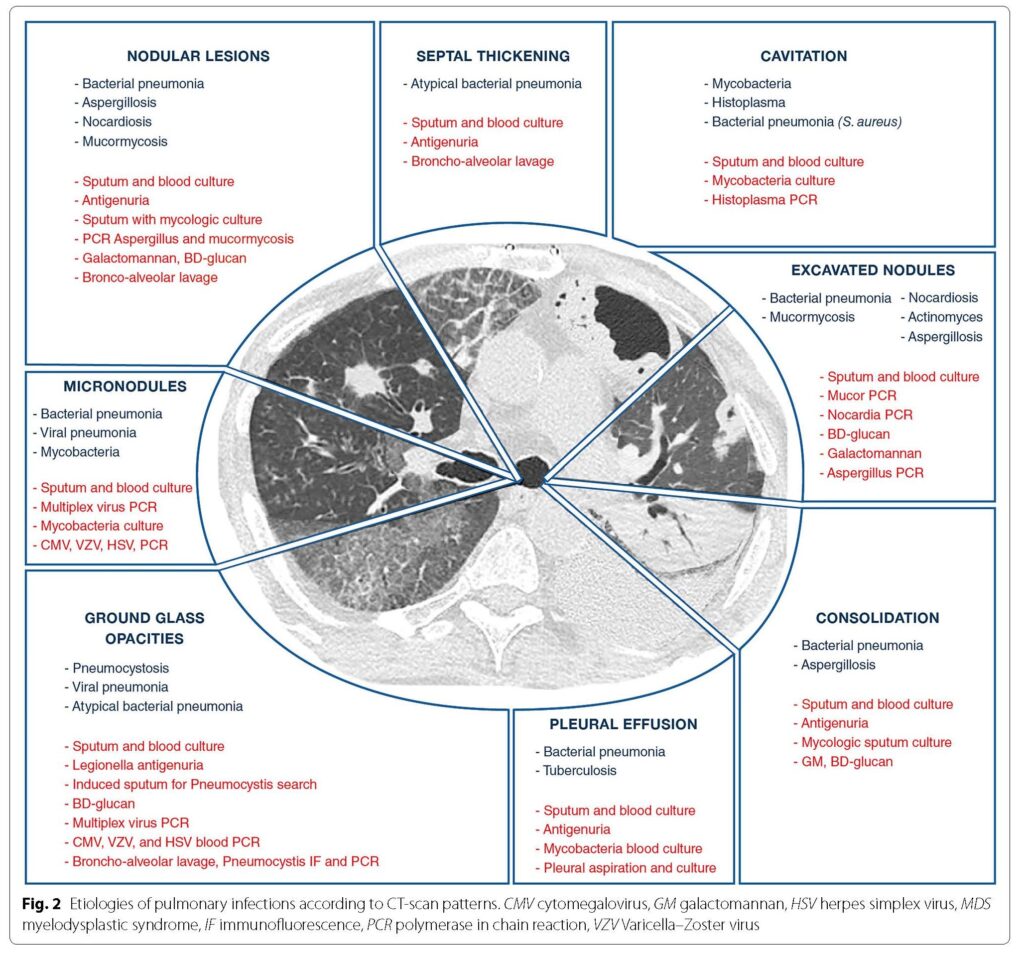
The episode also discussed radiographic features that are suggestive of invasive pulmonary fungal infections. Here are a few notes:
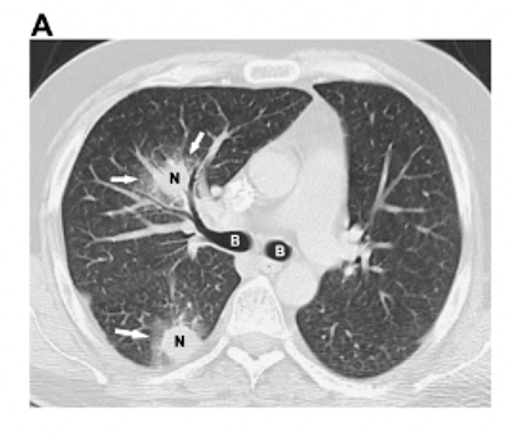
- Halo sign: CT finding of ground-glass opacity surrounding a pulmonary nodule or mass
- Most commonly associated with invasive pulmonary aspergillosis (25-95% incidence in neutropenic patients)
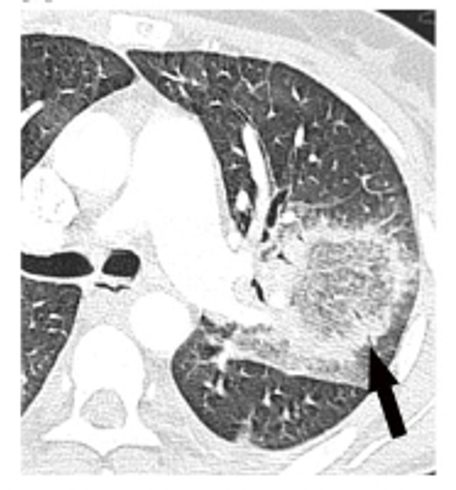
- Reverse halo (atoll) sign: focal rounded area of ground-glass opacity surrounded by a crescent or complete ring of consolidation
- Most commonly associated with pulmonary mucormycosis cases (19%)
- Calcification can be a sign of prior granulomatous infection and would need to consider dimorphic fungi (Coccidioides, Histoplasma, Blastomyces – depending on where you practice)
- Jessica also discussed the non-ID differential, such as rheumatologic (sarcoidosis, granulomatosis with polyangiitis), cryptogenic organizing pneumonia, malignancy
Some resources for further reading (in addition to those in the prior section):
- Boitsios G, Bankier AA, Eisenberg RL. Diffuse pulmonary nodules. AJR Am J Roentgenol. 2010;194(5):W354-W366. doi:10.2214/AJR.10.4345
- Georgiadou SP, Sipsas NV, Marom EM, Kontoyiannis DP. The diagnostic value of halo and reversed halo signs for invasive mold infections in compromised hosts. Clin Infect Dis. 2011;52(9):1144-1155. doi:10.1093/cid/cir122
Non-invasive evaluation for pulmonary nodules
Here is a chart that Ralph and Jessica provided for the Consult Notes:
Test | Sensitivity | Specificity | Comments |
1,3-B-D-glucan | 33-100% | 36-94% |
|
Aspergillus galactomannan (serum) | 61-79% | 81-95% |
|
Aspergillus galactomannan (BAL) | 58-90% | 84-96% |
|
Mold PCR plasma/whole blood | 84% (Aspergillus 79%) | 76% (Aspergillus 79.6%) |
|
BAL PCR | 77.2% | 93.5% |
|
Biopsy PCR | 89% | 100% | |
Blood Aspergillus Antibody by EIA | 67%-84% | 52%-67% |
|
As mentioned on the show, don’t forget to send specific testing in patients where relevant (such as Cryptococcal antigen or endemic fungi Ab/Ag testing)
Another quick reference would be the Febrile fungal markers chart (which is just focused on BDG and GM)
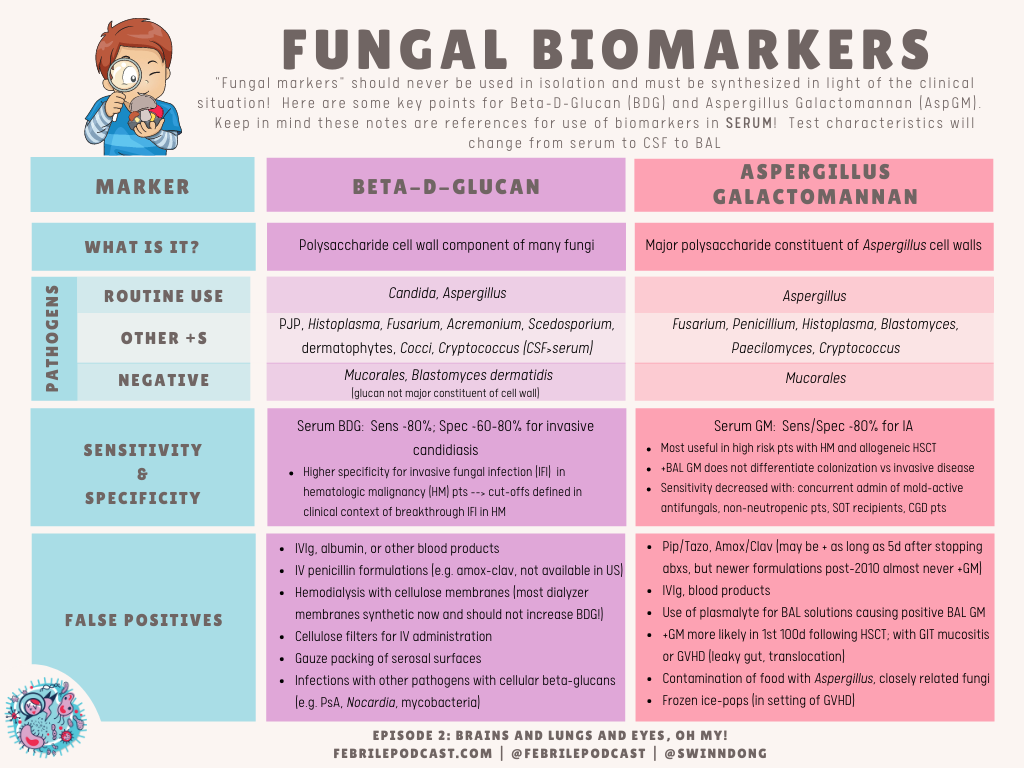
There are a ton of readings for fungal markers, and many references for the two tables above:
- A newer paper with more comprehensive overview of non-invasive testing for invasive fungal disease: Thompson GR 3rd, Boulware DR, Bahr NC, et al. Noninvasive Testing and Surrogate Markers in Invasive Fungal Diseases [published correction appears in Open Forum Infect Dis. 2022 Jul 28;9(7):ofac369]. Open Forum Infect Dis. 2022;9(6):ofac112. Published 2022 Mar 4. doi:10.1093/ofid/ofac112
- White PL, Alanio A, Brown L, et al. An overview of using fungal DNA for the diagnosis of invasive mycoses. Expert Rev Mol Diagn. 2022;22(2):169-184. doi:10.1080/14737159.2022.2037423
- Freeman Weiss Z, Leon A, Koo S. The Evolving Landscape of Fungal Diagnostics, Current and Emerging Microbiological Approaches. J Fungi (Basel). 2021;7(2):127. Published 2021 Feb 9. doi:10.3390/jof7020127
- Estimates of sensitivity and specificity were 79.2% (95% confidence interval (CI) 71.0 to 85.5) and 79.6% (95% CI 69.9 to 86.6) for a single positive test result, and 59.6% (95% CI 40.7 to 76.0) and 95.1% (95% CI 87.0 to 98.2) for two consecutive positive test results. : Cruciani M, Mengoli C, Barnes R, et al. Polymerase chain reaction blood tests for the diagnosis of invasive aspergillosis in immunocompromised people. Cochrane Database Syst Rev. 2019;9(9):CD009551. Published 2019 Sep 3. doi:10.1002/14651858.CD009551.pub4
- Arvanitis M, Ziakas PD, Zacharioudakis IM, Zervou FN, Caliendo AM, Mylonakis E. PCR in diagnosis of invasive aspergillosis: a meta-analysis of diagnostic performance. J Clin Microbiol. 2014;52(10):3731-3742. doi:10.1128/JCM.01365-14
- Sensitivity and specificity values (CIs) were 77.2% (62 to 87.6%) and 93.5% (90.6 to 95.6%), respectively: Avni T, Levy I, Sprecher H, Yahav D, Leibovici L, Paul M. Diagnostic accuracy of PCR alone compared to galactomannan in bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis: a systematic review. J Clin Microbiol. 2012;50(11):3652-3658. doi:10.1128/JCM.00942-12
- Karageorgopoulos DE, Vouloumanou EK, Ntziora F, Michalopoulos A, Rafailidis PI, Falagas ME. β-D-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis. 2011;52(6):750-770. doi:10.1093/cid/ciq206
- Lamoth F, Cruciani M, Mengoli C, et al. β-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3). Clin Infect Dis. 2012;54(5):633-643. doi:10.1093/cid/cir897
- Szyszkowitz A, Zurl C, Herzeg A, et al. Serum 1,3-Beta-D-Glucan Values During and After Laparoscopic and Open Intestinal Surgery. Open Forum Infect Dis. 2018;5(12):ofy296. Published 2018 Nov 8. doi:10.1093/ofid/ofy296
- Mennink-Kersten MA, Warris A, Verweij PE. 1,3-beta-D-glucan in patients receiving intravenous amoxicillin-clavulanic acid. N Engl J Med. 2006;354(26):2834-2835. doi:10.1056/NEJMc053340
- Mennink-Kersten MA, Ruegebrink D, Verweij PE. Pseudomonas aeruginosa as a cause of 1,3-beta-D-glucan assay reactivity. Clin Infect Dis. 2008;46(12):1930-1931. doi:10.1086/588563
- Marty FM, Koo S. Role of (1–>3)-beta-D-glucan in the diagnosis of invasive aspergillosis. Med Mycol. 2009;47 Suppl 1:S233-S240. doi:10.1080/13693780802308454
- William F. Wright, DO, MPH, Sue B. Overman, MA, SM(ASCP), Julie A. Ribes, MD, PhD, (1–3)-β-D-Glucan Assay: A Review of its Laboratory and Clinical Application, Laboratory Medicine, Volume 42, Issue 11, November 2011, Pages 679–685, https://doi.org/10.1309/LM8BW8QNV7NZBROG
- Tran T, Beal SG. Application of the 1,3-β-D-Glucan (Fungitell) Assay in the Diagnosis of Invasive Fungal Infections. Arch Pathol Lab Med. 2016;140(2):181-185. doi:10.5858/arpa.2014-0230-RS
- Miceli MH, Maertens J. Role of Non-Culture-Based Tests, with an Emphasis on Galactomannan Testing for the Diagnosis of Invasive Aspergillosis. Semin Respir Crit Care Med. 2015;36(5):650-661. doi:10.1055/s-0035-1562892
- Leeflang MM, Debets-Ossenkopp YJ, Wang J, et al. Galactomannan detection for invasive aspergillosis in immunocompromised patients. Cochrane Database Syst Rev. 2015;2015(12):CD007394. Published 2015 Dec 30. doi:10.1002/14651858.CD007394.pub2
Jessica also discussed invasive diagnostics (BAL with or without biopsy) as well as the criteria for proven/probable invasive fungal disease
- Proven invasive fungal disease – 1 of 4 criteria
- Histopathologic, cytopathologic, or direct microscopic examination of specimen obtain by FNA or biopsy with hyphae or yeast forms identified
- Culture of mold from a sterile site (excludes BAL)
- Blood culture that yields a mold
- Tissue – PCR amplification when fungal elements seen on formalin-fixed paraffin embedded tissue
- Probable invasive fungal disease
- Includes culture + or PCR + for mold from BAL fluid
- Wide range of sensitivity and specificity depending on the sample and diagnostic test
- 30-60% BAL alone +cultures
- Biopsy – culture + up to 78% if fungal elements seen on histopathology
- PCR sensitivity from biopsy in proven IFD
- 71% for open biopsy
- 50% core-needle biopsy
- 83% for body fluids
- Resources:
- Donnelly JP, Chen SC, Kauffman CA, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71(6):1367-1376. doi:10.1093/cid/ciz1008
- Gomez CA, Budvytiene I, Zemek AJ, Banaei N. Performance of Targeted Fungal Sequencing for Culture-Independent Diagnosis of Invasive Fungal Disease. Clin Infect Dis. 2017;65(12):2035-2041. doi:10.1093/cid/cix728
- Rickerts V, Mousset S, Lambrecht E, et al. Comparison of histopathological analysis, culture, and polymerase chain reaction assays to detect invasive mold infections from biopsy specimens. Clin Infect Dis. 2007;44(8):1078-1083. doi:10.1086/512812
Infographics
Goal
Listeners will be able to discuss the use of noninvasive fungal markers when investigating suspected pulmonary fungal infection.
Learning Objectives
After listening to this episode, listeners will be able to:
- Recognize radiographic features that are suggestive of invasive fungal infection
- Compare and contrast 1,3-beta-D-glucan, Aspergillus galactomannan, and mold-specific PCR
Disclosures
Our guests as well as Febrile podcast and hosts report no relevant financial disclosures
Citation
Ferguson, J., Tayyar, R., Dong, S. “#69: Investigating IFI”. Febrile: A Cultured Podcast. https://player.captivate.fm/episode/11924671-c113-4193-a686-7de383b3cb6c