Table of Contents
Credits
Hosts: Rita Dib, Sara Dong
Guest: Joseph Sassine
Writing: Rita Dib, Joseph Sassine
Producing/Editing/Cover Art/Infographics: Sara Dong
Our Guests
Guest Co-Host
Rita Dib, MD

Rita Wilson Dib is a chief Infectious Diseases fellow at University of Texas Health Science Center (UTHealth) and MD Anderson Cancer Center in Houston, TX, where she is focusing on oncology ID. She previously completed her internal medicine residency at the Medical College of Georgia.
Guest Discussant
Joseph Sassine, MD

Joseph Sassine, MD, FACP is an Assistant Professor of Medicine and Transplant ID Physician at the University of Oklahoma Health Sciences Center, as well as the ID fellowship associate program director. He previously completed his internal medicine residency at the Icahn School of Medicine at Mount Sinai – St. Luke’s-Roosevelt Hospital Center in New York City, and his infectious diseases fellowship at the University of Texas Health Science Center and MD Anderson Cancer Center in Houston. His research interests include viral infections in patients with hematological malignancies, including recipients of hematopoietic cell transplantation and cellular therapies.
Culture
Rita: Our Iceberg is Melting: Changing and Succeeding Under Any Conditions by John Kotter
Joseph: Ending Tuberculosis in the Face of Antimicrobial Resistance by Andrea Prinzi (on asm.org)
Consult Notes
Case Summary
55 yo man with relapsed DLBCL who going to receive CAR-T therapy
Key Points
What is CAR-T cell therapy and what are the commercially available FDA-approved products? What are the approved indications for CAR-T cell therapy?
- This episode featured commercially available, FDA-approved products – but will note that there is a broad range of other CAR-T, recombinant T cells, NK cells and other cellular therapy products that are still in early or late investigations for a wide variety of conditions/indications (that were not discussed)
- CAR-T stands for chimeric antigen receptor T cells
- The basic principle for chimeric antigen receptors is to engineer this receptor in order to graft defined specificity onto an immune effector cell and augment its function, and here we are specifically looking at its function against tumor cells.
- The structure of the chimeric antigen receptor closely replicates that of the T-cell receptor that is naturally present on T cells
- The first-generation CAR includes a T-cell activating domain and an extracellular immunoglobulin-derived heavy and light chain that is used to direct specificity
- The second-generation receptors and onwards add a co-stimulatory receptor to enhance the immune response, particularly enhancing proliferation and antiapoptotic function, and allowing direct expansion of the cells upon repeated exposure to the antigen that will occur in vivo. This is why we can refer to these cells as a persistent “living drug”
- Once these CAR T-cells are infused into the peripheral bloodstream, the cells will engraft and undergo extensive proliferation in vivo
- Then they will make their way towards tumor cells and will identify them by recognition of tumor antigens on the surface of the tumor cells by the chimeric antigen receptor on the surface of the T cells >> this will lead to death of the cancer cell and antigen release
- Through these functions, the CAR-T cells are able to promote immune surveillance to prevent recurrence
- The antigen release after the death of the cancer cells will be used by antigen presenting cells to prime and activate endogenous T cells that are already present and infiltrating the tumor, thus enhancing the endogenous immune response against the tumor.
- Additionally, the CAR T-cells will persist and expand in vivo thus allowing a positive feedback loop on the cycle of tumor recognition, depth of cancer cell, antigen release, and priming of endogenous T cells.
- For a good primer about CAR T-cells, Joseph recommended this review in the New England Journal of Medicine (published 2018)
- Currently, there are 6 FDA approved CAR-T cell products
- Four of these products are approved for relapsed/refractory B-cell malignancies including lymphoma, and for some products, acute lymphoblastic leukemia >> these products target the CD19 antigen, which is specific for B cells
- Two later products have been recently approved for relapsed/refractory multiple myeloma >> these products target the BCMA antigen which is specific for plasma cells.
| Product Name | Abbreviation | Target | Indication | Approval Year |
| Tisagenlecleucel | tisa-cel | CD19 | R/R B-ALL, R/R LBCL | 2017 |
| Axicabtagene cileucel | axi-cel | CD19 | R/R LBCL (incl. as 2nd line), R/R FL | 2017 |
| Brexucabtagene autoleucel | brex-cel | CD19 | R/R B-ALL, R/R MCL | 2020 |
| Lisocabtagene maraleucel | liso-cel | CD19 | R/R LBCL | 2021 |
| Idecabtagene vicleucel | ide-cel | BCMA | R/R MM | 2021 |
| Ciltacabtagene autoleucel | cilta-cel | BCMA | R/R MM | 2022 |
How are CAR-T cell products prepared?
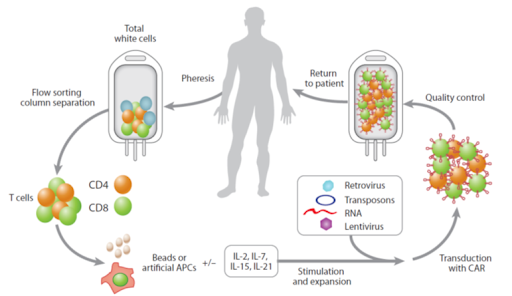
- Commercially available CAR-T’s are autologous products meaning that the cells are actually originating from the patient >> the cells are collected from the patient by apheresis
- In the lab, those cells are transduced with a CD19 or BCMA chimeric antigen receptor retroviral or lentiviral vector to insert a copy of the DNA that encodes for chimeric antigen receptor into the genome of the T cells
- There is also an alternative non-viral vector method by electroporation
- After transduction:
- cells undergo expansion and purification ex vivo, with the help of specific cytokines and interleukins
- they get tested for quality and sterility
- they are cryopreserved and shipped to the patient
- This process is estimated to take about 2 to 4 weeks, sometimes 6 weeks, and it is important for us to realize that during this time, the patient is either waiting for the cells without any chemotherapy, or is receiving bridging chemotherapy.
- Check out these resources reviewing CAR-T as well:
- Shank BR, Do B, Sevin A, Chen SE, Neelapu SS, Horowitz SB. Chimeric Antigen Receptor T Cells in Hematologic Malignancies. Pharmacotherapy. 2017;37(3):334-345. doi:10.1002/phar.1900
- Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annu Rev Med. 2014;65:333-347. doi:10.1146/annurev-med-060512-150254
- Joseph explained why this preparation method is relevant to us in ID: The transduction of the CAR-T gene into the T-cell relies on a lentiviral/retroviral vector, so there have been multiple case reports of patients screening positive on HIV nucleic acid antigen test after CAR-T cell
- The first case report was from MD Anderson in 2017, a patient underwent CD19 CAR-T for follicular lymphoma, and was being planned for a hematopoietic cell transplant for consolidation 2 months later. As part of their pre-HCT screening, the patient was tested for HIV using a nucleic acid antigen test, and that screening test returned positive. The quantitative HIV RNA was detectable at 74 copies per mL, the fourth-generation HIV screen was negative, and the patient’s CD4 count was 837. It turns out that the CAR T-cells were actually created using a lentiviral vector, which contained HIV gene sequences including LTR and gag, and depending on which assay you used to detect and quantify HIV, 2 of the most commonly used assays actually use LTR or gag or both as target sequences.
- Since then, there have been multiple reports of a similar scenario, most of which involves the COBAS AmpliPrep/COBAS TaqMan HIV-1 test which uses both LTR and gag as target sequences.
- So back to the CAR-T world: meanwhile as the cells are being prepared, the patient might be receiving bridging chemotherapy (which depends on the nature and the status of the disease) >> this chemotherapy is usually administered after apheresis to stabilize the disease while the CAR-T cells are manufactured
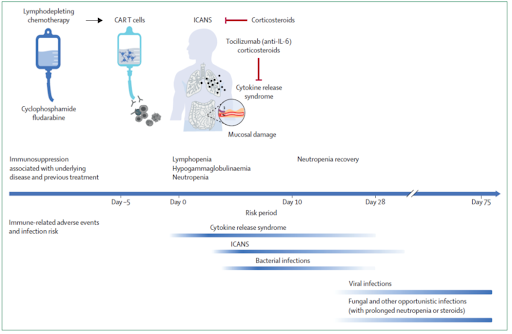
- Once the CAR-T cells are available, the patient then receives lymphodepleting chemotherapy (the most commonly used regimen nowadays is fludarabine and cyclophosphamide), which is administered before infusion of the CAR-T cells, usually a few days before
- The goal here is to deplete the T, B and NK cells and create a favorable environment for expansion and persistence of the CAR T-cells in vivo
- Just like in hematopoietic cell transplant, we refer to the day of CAR-T cell infusion is day 0
- While this entire process (since lymphodepletion followed by cell infusion) has traditionally happened in the inpatient setting, there is an increasing trend towards moving the entire CAR-T process to the outpatient setting, with good success in terms of safety.
- Borogovac A, Keruakous A, Bycko M, et al. Safety and feasibility of outpatient chimeric antigen receptor (CAR) T-cell therapy: experience from a tertiary care center. Bone Marrow Transplant. 2022;57(6):1025-1027. doi:10.1038/s41409-022-01664-z
What are the risk factors for infection in recipients of CAR-T therapies? Is there a way to risk stratify these patients?
- Joseph approached the risk factors for infection in CAR-T cell recipients by differentiating into 3 stages, risk factors that:
- Exist before CAR-T
- During CAR-T (which also includes early period or the first 30 days after CAR-T)
- Long-term risk factors beyond 30 days after CAR-T
- Risk factors before CAR-T
- Mostly talking about host related risk factors here
- Underlying disease
- Status of disease
- Previous therapies received (both in terms of cumulative immunosuppression and in terms of specific lines of therapy)
- Baseline cytopenias
- Other medical comorbidities
- History of prior infections
- Mostly talking about host related risk factors here
- Risk factors during CAR-T and within the early period after treatment
- Dose of cells being infused
- Conditioning regimen being used
- Early complications, such as
- An initial period of neutropenia
- Cytokine release syndrome (CRS)
- Immune effector cell associated neurotoxicity syndrome (ICANS)
- Hemophagocytic lymphohistiocytosis (HLH) / Macrophage activation syndrome (MAS)
- Risk factors beyond 30 days
- Prolonged neutropenia
- Prolonged B-cell aplasia with subsequent hypogammaglobulinemia
- Need for more prolonged courses of steroids to treat ICANS
- Primary disease relapses requiring additional immunosuppression
- Joseph mentioned a review paper from the Lancet Haematology (2021), which describes risk for infection and identifies the patients at highest risk for infection at the beginning of or during CAR-T cell therapy as those with relapsed or refractory lymphoid malignancy, extensive pretreatment with previous chemotherapy with or without glucocorticoids, previous allogeneic HCT, previous invasive mold disease, active CMV disease, CRS or HLH or ICANS requiring anti-IL-6 or glucocorticoid treatment, baseline neutropenia or prolonged neutropenia post CAR-T cell, or baseline lymphopenia
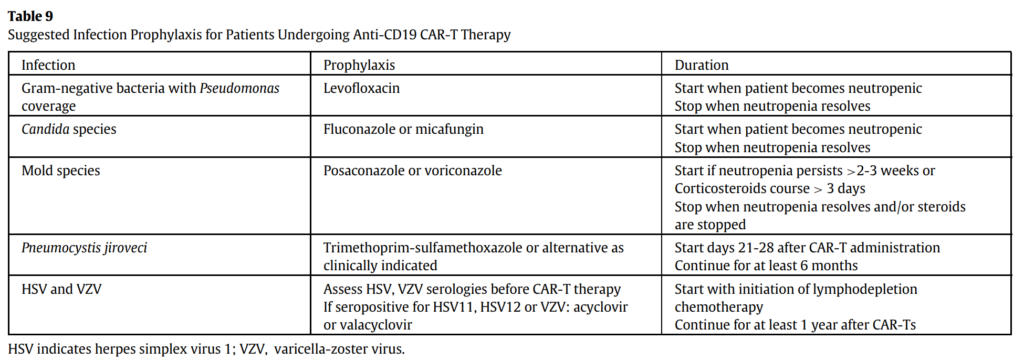
How do we plan a prophylactic regimen for these patients?
- In terms of prophylaxis, we do not have clinical trial data to specifically inform prophylactic strategies and recipients of CAR T-cells. Thus, we rely on expert opinion and extrapolation from hematopoietic cell transplant, where we have stronger evidence to support specific prophylactic strategies
- Additionally, we are informed by the prophylactic strategies that have been used in the now published retrospective or prospective cohort studies from large cancer centers
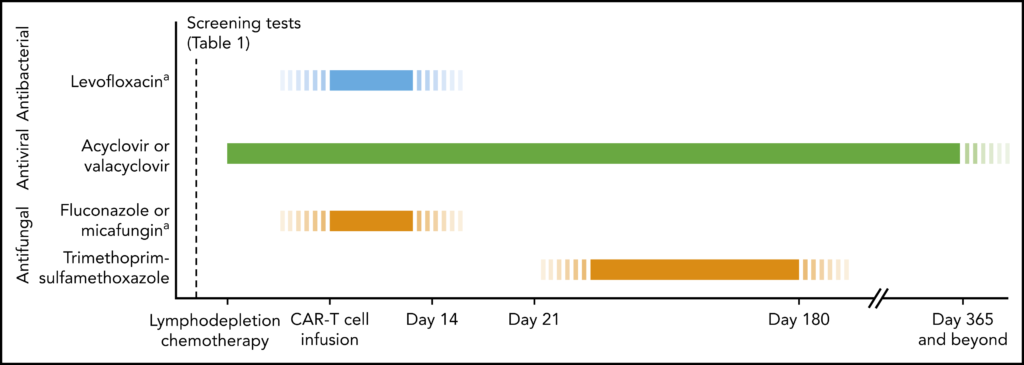
- For example, the overwhelming majority of the studies had patients on HSV/VZV prophylaxis and pneumocystis prophylaxis, whereas antibacterial prophylaxis and antifungal prophylaxis has been very variable among centers
- In general, for bacterial infections, the common strategy would be fluoroquinolone prophylaxis, usually with levofloxacin, during periods of neutropenia, as is the case with hematopoietic cell transplant recipients in patients with leukemia and prolonged neutropenia
- For HSV and VZV prophylaxis there is a broad consensus to give acyclovir or valacyclovir from initiation of lymphodepletion and for at least 1 year after CAR-T
- In terms of antifungals, the general recommendation for yeast prophylaxis (meaning essentially Candida), would be fluconazole or micafungin during periods of neutropenia (mold active prophylaxis will be discussed later)
- Finally, for pneumocystis prophylaxis this is indicated from about day 21 and up until at least 6 months after CAR-T.
One of the very first papers to discuss this is a how I treat, or rather how I prevent, paper published in Blood in 2020: Hill JA, Seo SK. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood. 2020 Aug 20;136(8):925-935. doi: 10.1182/blood.2019004000. PMID: 32582924; PMCID: PMC7441168.
The ASTCT does address antimicrobial prophylaxis in their CAR-T for relapsed/refractory B-cell lymphoma guideline from 2019.
The most recent iteration of the NCCN guidelines for prevention and treatment of cancer related infections in October 2022 also has a section about prophylaxis and CAR-T cell recipients. Guidelines Detail (nccn.org)
The patient in the episode had fever, which is a common ID consult after CAR-T. Here are some notes from how Joseph approached the case:
- The infusion of the CAR-T status is usually preceded by lymphodepleting chemotherapy, most commonly fludarabine and cyclophosphamide, and this treatment results in neutropenia
- This neutropenia is expected to resolve by day 14 after CAR-T, beyond which this would be considered a prolonged neutropenia
- During this time period, the immunophysiology resembles that of a hematopoietic cell transplant recipient, with neutropenia and mucositis being the main drivers of infection
- These patients commonly have central venous catheters as well
- So, from an infection perspective, you are looking mostly at infections that happen in neutropenic patients, such as bloodstream infections, caused by bacterial organisms or by Candida
- These bloodstream infections are related to either translocation across mucosal barrier injury, or to the presence of central venous catheters
- You can also see other types of bacterial infections:
- Skin and soft tissue infection
- Neutropenic enterocolitis
- Bacterial pneumonia
- Respiratory viral infection
- Or recurrence of infection that patient recently had prior to CAR-T
- Joseph started the approach like you would for usual work-up for febrile neutropenia, including:
- Blood cultures
- Chest x-ray
- Respiratory viral panel
- Additional work-up driven by signs and symptoms on history and physical exam
- Initiation of an empirical antipseudomonal beta-lactam, such as cefepime or piperacillin/tazobactam, is recommended in this case. In certain patients, and depending on the syndrome being suspected, adding a second agent with MRSA activity might be warranted.
- More often than not, we are unable to determine a specific source of infection, or to establish a microbiological diagnosis for the neutropenic fever
- This could be because of transient GI translocation causing transient bacteremia
- However, we also need to consider the early complications of CAR-T which can also manifest with neutropenic fever.
What are some early complications of CAR-T that can manifest with neutropenic fever?
- The first of these is cytokine release syndrome or CRS
- This is a syndrome triggered by activation of T cells upon engagement of the chimeric antigen receptors with the antigens expressed by tumor cells, leading to the release of cytokines and chemo kinds by activated T cells, such as interleukin-2, interferon gamma, interleukin-6 and GM-CSF, accompanied with the release of cytokines by bystander immune cells such as interleukin-6, 8 and 10
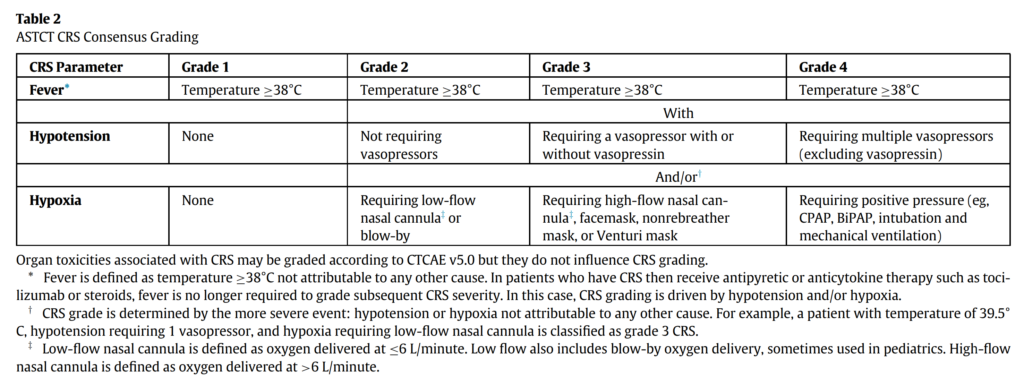
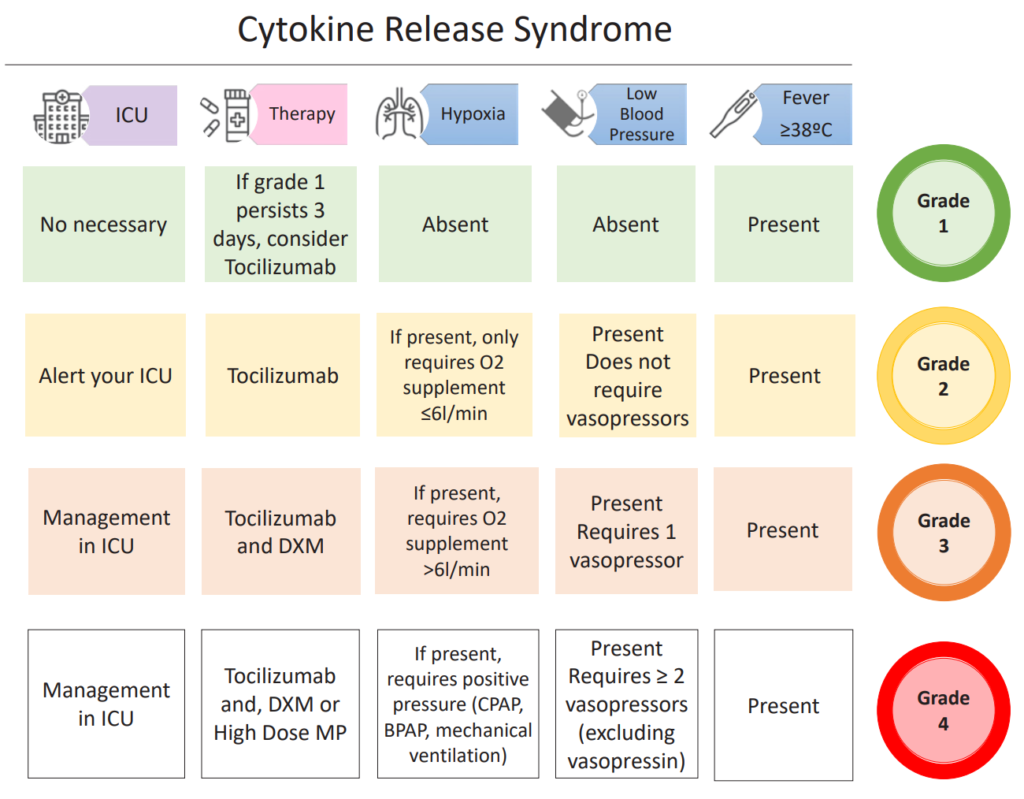
- This can lead to symptoms that can virtually affect any organ system, with the most common symptoms being fever, fatigue, tachycardia, hypotension, as you would expect any cytokine storm to present as
- Onset of CRS is variable; however, it peaks around 2 to 7 days after infusion, but can still present up to 3 weeks after CAR-T
- Depending on the studies, the incidence varies between 57 and 93%
- The 2019 ASTCT guidelines offer a consensus grading system for CRS, that relies on 3 parameters: fever, hypotension (vasopressor requirement), and hypoxia, depending on the extent of oxygen requirement)
- The management of CRS depends on the grade, but usually involves tocilizumab, possibly corticosteroids for high-grade CRS, and ICU management if vasopressors and/or mechanical ventilation are required.
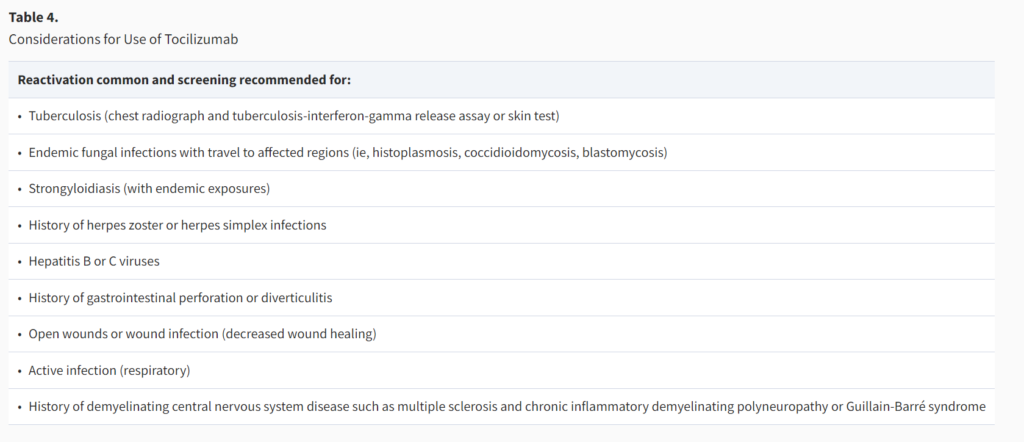
- **Keep in mind that tocilizumab is a powerful immunosuppressant
- It has been recently used extensively in COVID-19 but in most of these patients, it was given as a one-time dose
- The largest extent of data we have on the use of tocilizumab is chronic use in patients with rheumatological diseases
- There is increased risk for reactivation of certain infections after the use of tocilizumab, including tuberculosis, endemic fungal infections, strongyloidiasis, herpes zoster or herpes simplex, hepatitis B or C
- Tocilizumab has also been associated with decreased wound healing and increased risk for GI perforation
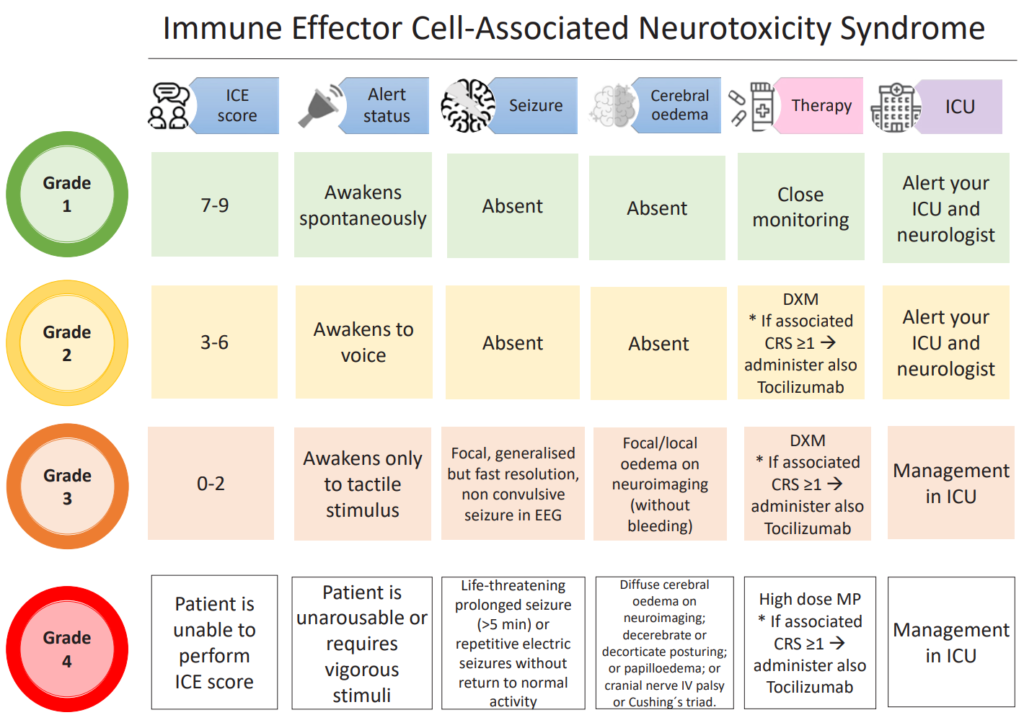
- The other important complication in the early period after CAR-T is immune effector cell associated neurotoxicity syndrome or ICANS
- There are 2 potential explanations for why this happens, including passive diffusion of cytokines into the brain and/or trafficking of T cells into the CNS
- It occurs at the median time of 4 days after infusion, with an incidence ranging between 20 and 70%
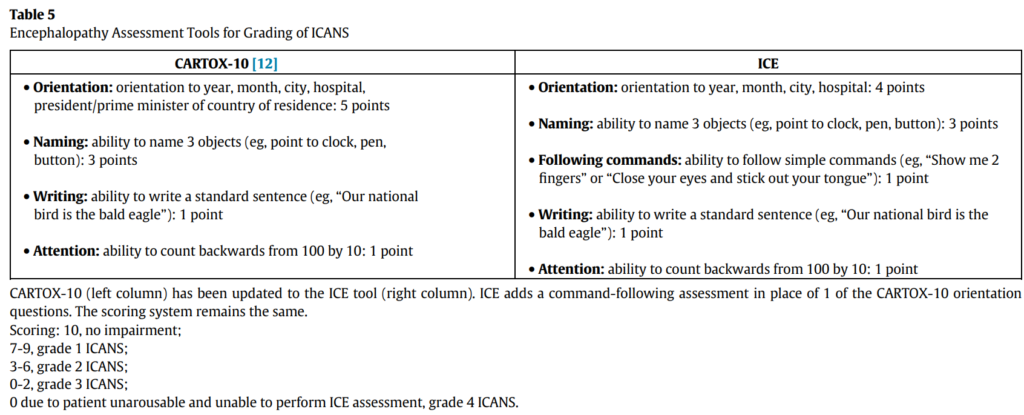
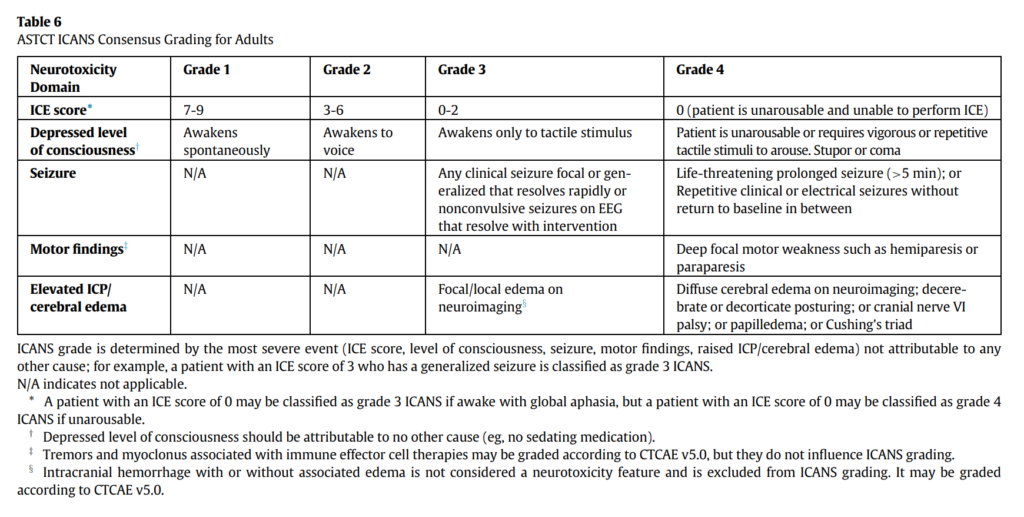
- ICANS grading per the ASTCT guidelines relies on 5 domains of neurotoxicity, including level of consciousness, seizure, motor findings, elevated intracranial pressure/cerebral edema, and the ICE score which evaluates orientation, naming, following commands, writing and attention
- ICANS is also graded from grade 1 through grade 4
- The management of ICANS depends on the grade, the cornerstone of pharmacological management includes high-dose glucocorticoids, often with prolonged tapers, and might also involve ICU admission.
- Another early complication, but fortunately less common, is hemophagocytic lymphohistiocytosis and macrophage activation syndrome HLH/MAS, which essentially represents the extreme end of the CRS spectrum
- Occurs with an incidence of 1 to 3.5%
- Diagnosis relies on meeting certain criteria including a peak serum ferritin level of more than 10,000 ng/mL during the CRS phase, typically the first 5 days, subsequently developing any 2 of the following, including increase in liver function test, acute kidney injury, pulmonary edema, or evidence of hemophagocytosis in bone marrow or organs
- The initial management includes anti-IL-6 therapy with corticosteroids just like any severe CRS, and if no improvement after 48 hours, addition of etoposide, and possibly intrathecal cytarabine for neurotoxicity.
What infections commonly occur early after receipt of CD19 CAR-T therapy?
- There is no clear definition of what early or late means, but when we say early we assume in the first 30 to 90 days after CAR-T
- Most of what we know about infections after CAR-T is informed by large cohort studies, some retrospective and some prospective, done at the major cancer centers in the US. So, we will review quickly some of the studies and try to gauge a pattern of infections from this data
- The first study was published out of Fred Hutch in Blood in 2018: Hill JA, Li D, Hay KA, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. 2018;131(1):121-130. doi:10.1182/blood-2017-07-793760
- Included 133 adult patients with CD19 positive ALL, CLL or B-cell lymphoma enrolled in phase 1/2 trial of CD19 CAR-T cells
- These patients received antimicrobial prophylaxis including for HSV/VZV, bacteria, yeast, and pneumocystis and were followed up until day 90
- Of this cohort, 16.5% developed bacterial infections: half of which were bloodstream infections, 8% developed viral infections (most of which were respiratory viruses), and 3% developed fungal infections
- The first 28 days, most of the infections were bacterial, whereas viral infections were the dominant infections between day 29 and day 90
- There were more severe and life-threatening/fatal infections in the first 28 days
- In the univariate model, the risk factors for infection within the first 28 days were a higher CAR-T cell dose, CRS, ICANS, use of tocilizumab and ICU admission
- In the multivariate analysis, CRS was the only significant variable. The conditioning regimen of fludarabine and cyclophosphamide with optimized CAR-T cell dose was associated with a reduced risk of severe CRS and demonstrated a lower infection density compared to other experimental regimens.
- The next study was published out of Sloan-Kettering in CID in 2018: Park JH, Romero FA, Taur Y, et al. Cytokine Release Syndrome Grade as a Predictive Marker for Infections in Patients With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia Treated With Chimeric Antigen Receptor T Cells. Clin Infect Dis. 2018;67(4):533-540. doi:10.1093/cid/ciy152
- Included 53 adults with relapsed/refractory ALL on a phase 1 trial, almost all of whom received HSV/VZV, yeast and PCP prophylaxis and were followed for 180 days
- Bacterial infections occurred at a median of 18 days, fungal infections occurred at a median of 23 days, and viral infections at a median of 48 days
- In the first 30 days, 42% of the patients developed an infection, the overwhelming majority of which were bacterial infections, whereas respiratory viruses represented the overwhelming majority of infections beyond D30
- CRS grade 3 and above was the main predictor of infection and of bloodstream infection on multivariate analysis
- Another study out of Sloan-Kettering published in Blood Cancer Journal in 2020: Wudhikarn K, Palomba ML, Pennisi M, et al. Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma. Blood Cancer J. 2020;10(8):79. Published 2020 Aug 5. doi:10.1038/s41408-020-00346-7
- Followed 60 patients with relapsed refractory DLBCL receiving CD19 CAR-T for up to 1 year, almost all of whom got HSV/VZV, yeast and pneumocystis prophylaxis
- Also showed that in the first 30 days, 68% of the infections were bacterial with a median onset of 12 days, 28% of those infections were C. difficile, 20% of the patients developed a viral infection with immediate onset of 8 days, more than half of these were respiratory viruses
- One third of the infection events occurred during the index admission
- Predictors for infection on multivariate analysis were systemic steroids, predictors for severe bacterial infection where impaired performance status and previous infection 30 days before lymph depletion
- They did notice a similar incidence of infection before and after implementation of the standardized antimicrobial prophylaxis guidelines.
- One more cohort study, this time out of Moffitt, published in 2021: Logue JM, Zucchetti E, Bachmeier CA, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica. 2021;106(4):978-986. Published 2021 Apr 1. doi:10.3324/haematol.2019.238634
- 85 patients with relapsed refractory large B-cell lymphoma receiving CD19 CAR-T, with a universal antimicrobial prophylaxis strategy
- 36% of the patients develop an infection in the first 30 days, 39% of these were C diff, 35% of the infections were severe
- The highest incidence rate of infection was noted in the first 30 days with an incidence rate of 11.7 per 1000 person-days
- This drops to 2.3 between day 30-day 90 then consistently below 1 past day 90
- Severe infection before day 30 was associated with severe CRS, severe neurotoxicity, use of tocilizumab or steroids, and need for bridging therapy before CAR-T.
- Another study of CD19 CAR-T and early infections out of Fred Hutch: Vora SB, Waghmare A, Englund JA, Qu P, Gardner RA, Hill JA. Infectious Complications Following CD19 Chimeric Antigen Receptor T-cell Therapy for Children, Adolescents, and Young Adults. Open Forum Infect Dis. 2020;7(5):ofaa121. Published 2020 Apr 9. doi:10.1093/ofid/ofaa121
- This time including pediatric patients and young adults ages 1 through 26 years, with only pneumocystis prophylaxis and very variable other antimicrobial prophylaxis (no bacterial prophylaxis)
- Again showed the highest density of infections in the first 28 days
- Most of the bacterial infections occurred in patients with neutropenia, whereas viral infections occurred equally in periods of neutropenia or without neutropenia
- Of the 3 CAR-T cell infusion factors, the use of fludarabine/cyclophosphamide was associated with a lower infection density compared to other level depleting regimens
- This is likely related to the use of 0.5 g/m² of cyclophosphamide in Flu/Cy versus 3 g/m² of cyclophosphamide in other regimens
- Time to first infection was shorter and patient should receive prior HCT, in patients with hypogammaglobulinemia with an IgG less than 400 mg/dL, and was longer in patients with received lymphoid depletion with fludarabine and cyclophosphamide.
- Since then, there have been multiple additional cohort studies, following patients for longer durations up to 1 year, which we will review little bit later when we talk about late infections, but the overwhelming pattern with CD19 CAR-T is a predominance of bacterial infections in the first 30 days after CAR-T
- Those infections can be bloodstream infections, or bacterial site infection such as pneumonias, urinary, or skin and skin structure infections, some studies also showed a sizable proportion of C. difficile infections. These early infections tend to be more severe than infections occurring past 30 days. The major risk factor seems to be CRS +/- need for immunosuppression to treat CRS and ICANS, which occurs at the time of significant neutropenia.
Infographics
Goal
Listeners will be able to understand early complications after CAR-T therapy.
Learning Objectives
After listening to this episode, listeners will be able to:
- Describe the indications and preparation of CAR-T cell therapy
- Discuss early complications seen after receipt of CAR-T cell therapy that can manifest as neutropenic fever, including CRS and ICANS
- Identify infections that commonly occur within the first 30-90 days after CAR-T therapy
Disclosures
Our guests as well as Febrile podcast and hosts report no relevant financial disclosures.
As Joseph noted on the audio, this episode discusses commercially available, FDA-approved products (there are a broad range of other cellular therapy products that are still in investigation for a wide variety of conditions/indications)
Citation
Sassine, J., Dib, R., Dong, S. “#73: Fast & Feverish: Intro to CAR-T & Early ID Complications”. Febrile: A Cultured Podcast. https://player.captivate.fm/episode/8b18f7e6-eb9d-46d1-92d2-ad525e230fa3