Table of Contents
Credits
Host: Sara Dong
Guests: Efteraj Alhowity, Bashayer Alshehail
Writing/Producing/Editing/Cover Art: Sara Dong
Our Guests

Dr. Bashayer Alshehail
Dr. Bashayer Alshehail is an infectious disease clinical pharmacist consultant at King Fahad Hospital of the University in al Khobar and an assistant professor at pharmacy practice department at IAU. She has a PGY1 in clinical pharmacy and a PGY2 specialty in infectious diseases. Furthermore, she is a board-certified infectious disease pharmacist and chairperson of the ID specialty group in the Saudi Society of Clinical Pharmacy.
Dr. Efteraj Alhowity
Dr. Efteraj Alhowity is a Pediatric Infectious Disease Consultant and Chairperson of antimicrobial stewardship committee at the King Salman Armed Forces Hospital in Tabuk, KSA. She completed her fellowship program in pediatric infectious diseases in Riyadh and is Saudi and Arab pediatric board certified.
Culture
Both of our guests recommended exploring some of the historical sites/architecture in Riyadh, including the Diriyah which is part of a UNESCO heritage site. Check out others in Saudi Arabia here: https://whc.unesco.org/en/statesparties/sa. Both of our guests traveled to Riyadh and were also visiting
Consult Notes
Consult Q
Please assist in management of pneumonia due to a very resistant Pseudomonas
Key Points
Welcome to a three episode series recorded live at the World Antimicrobial Resistance Awareness Week (WAAW) Forum held and organized by the Saudi Pediatric Infectious Diseases Society (SPIDS) in collaboration with Febrile and the King Abdulaziz Public Library
In addition to our guests that you’ll meet on episodes 86-88, a special thank you to:
- Dr. Rana Almaghrabi, President of SPIDS
- Dr. Fatimah Aldubisi, Head of Scientific and Research Committee
Key Resource
Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America Antimicrobial-Resistant Treatment Guidance: Gram-Negative Bacterial Infections. Infectious Diseases Society of America 2023; Version 3.0. Available at https://www.idsociety.org/practice-guideline/amr-guidance/.
The patient case
Teenager with history of cystic fibrosis and colonization with MDR-Pseudomonas who was admitted to hospital with respiratory failure
- She had a prolonged hospital course requiring ECMO support and ultimately underwent bilateral lung transplant
- Post-transplant she was struggled with recurrent MDR-Pseudomonas pulmonary infections requiring multiple long courses of antibiotics
- Before we dive further into the current case, I want to pull up a susceptibility report which notes resistance to ciprofloxacin/levofloxacin, imipenem/meropenem – but there is susceptibility to ceftazidime, cefepime, and piperacillin-tazobactam.
Isolate: Pseudomonas aeruginosa (aerobic bottle) | |||
Antibiotic | Intrp | MIC | Intrp |
Aztreonam | S | ||
Ceftazidime | S | ||
Ciprofloxacin | R | ||
Cefepime | S | ||
Gentamicin | S | ||
Imipenem | R | ||
Levofloxacin | R | ||
Meropenem | R | ||
Piperacillin/tazobactam | S | ||
Tobramycin | S | ||
Ceftazidime/avibactam | 4 | S | |
Colistin | 4 | R | |
Ceftolozane/tazobactam | 1 | S | |
The episode case isolate is carbapenem resistant Pseudomonas but why does it appear to still have susceptibility to cefepime and pip-tazo?
This is likely NOT carbapenemase mediated resistant but from OprD (a porin) downregulation
Some definitions/terminology of carbapenem resistance in Pseudomonas
- MDR-PsA is defined as Pseudomonas aeruginosa not susceptible to at least one antibiotic in at least 3 antibiotic classes for which PsA susceptibility is generally expected: penicillins, cephalosporins, fluoroquinolones, aminoglycosides, carbapenems
- Difficult-to-treat resistance (DTR-P.aeruginosa) is exhibiting non-susceptibility to all: piperacillin-tazobactam, ceftazidime, cefeipme, aztreonam, meropenem, imipenem-cilastatin, ciprofloxacin, levofloxacin
What are the mechanisms of carbapenem resistance in Pseudomonas?
- MDR and DTR-PsA generally evolve as result of interplay of multiple complex resistance mechanisms including:
- Decreased expression of outer membrane porins (OprD)
- Increased production of or amino acid substitutions within Pseudomonas-derived cephalosporinase (PDC) enzymes (commonly referred to as pseudomonal AmpC enzymes)
- Tazobactam not effective against Pseudomonas derived cephalosporinases
- Upregulation of efflux pumps (eg MexAB-OprM)
- Mutations in penicillin-binding protein targets
- Presence of ESBLs (eg blaOXA-10)
- Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev. 2009;22(4):582-610. doi:10.1128/CMR.00040-09
- Wolter DJ, Lister PD. Mechanisms of β-lactam resistance among Pseudomonas aeruginosa. Curr Pharm Des. 2013;19(2):209-222.
- Qin S, Xiao W, Zhou C, et al. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct Target Ther. 2022;7(1):199. Published 2022 Jun 25. doi:10.1038/s41392-022-01056-1
Mechanisms of antimicrobial resistance in Pseudomonas aeruginosa table from Qin, et al. above
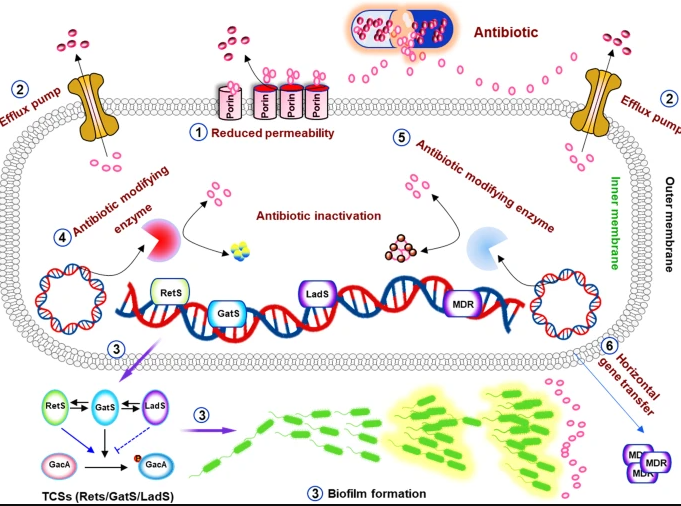
This is a good reminder that the term CRE for carbapenem-resistant Enterobacterales also represents a heterogenous mix of mechanisms – meaning CRE could be carbapenemase producing organisms or non-carbapenemase producing
Carbapenemase production is rare cause of carbapenem resistance in PsA in the US, but what about elsewhere in the world?
- Relatively rare in the US
- Identified in upwards of 20% of carbapenem-resistant PsA in other regions of the world, most commonly due to presence of blaVIM enzymes
- Reyes J, Komarow L, Chen L, et al. Global epidemiology and clinical outcomes of carbapenem-resistant Pseudomonas aeruginosa and associated carbapenemases (POP): a prospective cohort study. Lancet Microbe. 2023;4(3):e159-e170. doi:10.1016/S2666-5247(22)00329-9 → Prevalence of carbapenemase genes among CRPA isolates varied by region with south and central america 69%, Australia and Singapore 57%, China 32%, Middle East 30% [vs US 2%]
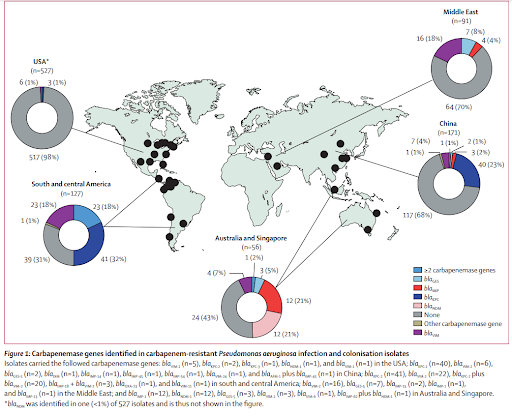
- Additional resources:
- Karlowsky JA, Kazmierczak KM, de Jonge BLM, Hackel MA, Sahm DF, Bradford PA. In Vitro Activity of Aztreonam-Avibactam against Enterobacteriaceae and Pseudomonas aeruginosa Isolated by Clinical Laboratories in 40 Countries from 2012 to 2015. Antimicrob Agents Chemother. 2017;61(9):e00472-17. Published 2017 Aug 24. doi:10.1128/AAC.00472-17
- Karlowsky JA, Kazmierczak KM, Bouchillon SK, de Jonge BLM, Stone GG, Sahm DF. In Vitro Activity of Ceftazidime-Avibactam against Clinical Isolates of Enterobacteriaceae and Pseudomonas aeruginosa Collected in Asia-Pacific Countries: Results from the INFORM Global Surveillance Program, 2012 to 2015. Antimicrob Agents Chemother. 2018;62(7):e02569-17. Published 2018 Jun 26. doi:10.1128/AAC.02569-17
- Escandón-Vargas K, Reyes S, Gutiérrez S, Villegas MV. The epidemiology of carbapenemases in Latin America and the Caribbean. Expert Rev Anti Infect Ther. 2017;15(3):277-297. doi:10.1080/14787210.2017.1268918
- Gill CM, Aktaþ E, Alfouzan W, et al. The ERACE-PA Global Surveillance Program: Ceftolozane/tazobactam and Ceftazidime/avibactam in vitro Activity against a Global Collection of Carbapenem-resistant Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 2021;40(12):2533-2541. doi:10.1007/s10096-021-04308-0
- We touched on epidemiology in Saudi Arabia / regionally in the Middle East
- Al-Orphaly M, Hadi HA, Eltayeb FK, et al. Epidemiology of Multidrug-Resistant Pseudomonas aeruginosa in the Middle East and North Africa Region. mSphere. 2021;6(3):e00202-21. Published 2021 May 19. doi:10.1128/mSphere.00202-21
- Hafiz TA, Bin Essa EA, Alharbi SR, et al. Epidemiological, Microbiological, and Clinical Characteristics of Multi-Resistant Pseudomonas aeruginosa Isolates in King Fahad Medical City, Riyadh, Saudi Arabia. Trop Med Infect Dis. 2023;8(4):205. Published 2023 Mar 30. doi:10.3390/tropicalmed8040205.
- Al-Orphaly M, Hadi HA, Eltayeb FK, et al. Epidemiology of Multidrug-Resistant Pseudomonas aeruginosa in the Middle East and North Africa Region. mSphere. 2021;6(3):e00202-21. Published 2021 May 19. doi:10.1128/mSphere.00202-21
- Al-Tawfiq JA, Rabaan AA, Saunar JV, Bazzi AM. Genotypes and prevalence of carbapenemase-producing Enterobacteriaceae and Pseudomonas aeruginosa in a hospital in Saudi Arabia. Trans R Soc Trop Med Hyg. 2022;116(1):50-53. doi:10.1093/trstmh/trab055
What are the preferred antibiotics for treatment of infections caused by MDR PsA?
In this case, we have a patient critically ill in the ICU with a PsA isolate resistant to carbapenems but susceptible to novel beta-lactam agent, would use one of these novel beta lactam-beta-lactamase inhibitor combinations (ceftolozane-tazobactam, ceftazidime-avibactam, imipenem-cilastatin-relebactam.
- This is sometimes not typically your first option because we hope to preserve effectiveness of novel BLBLIs for future infections, but in cases of treatment of serious infections outside of the urinary tract, this is the ideal option
- This is based on in vitro activity, observational studies, and most importantly clinical trial data – although majority of patients in clinical trials receiving these newer beta-lactam agents were not infected with DTR-Pseudomonas aeruginosa. No comparative studies between novel agents to guide treatment decisions (studies have compared to older agents like polymyxins)
- Carmeli Y, Armstrong J, Laud PJ, et al. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect Dis. 2016;16(6):661-673. doi:10.1016/S1473-3099(16)30004-4
- Motsch J, Murta de Oliveira C, Stus V, et al. RESTORE-IMI 1: A Multicenter, Randomized, Double-blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs Colistin Plus Imipenem in Patients With Imipenem-nonsusceptible Bacterial Infections. Clin Infect Dis. 2020;70(9):1799-1808. doi:10.1093/cid/ciz530
- Kollef MH, Nováček M, Kivistik Ü, et al. Ceftolozane-tazobactam versus meropenem for treatment of nosocomial pneumonia (ASPECT-NP): a randomised, controlled, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2019;19(12):1299-1311. doi:10.1016/S1473-3099(19)30403-7
- Torres A, Zhong N, Pachl J, et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis. 2018;18(3):285-295. doi:10.1016/S1473-3099(17)30747-8
- Mazuski JE, Gasink LB, Armstrong J, et al. Efficacy and Safety of Ceftazidime-Avibactam Plus Metronidazole Versus Meropenem in the Treatment of Complicated Intra-abdominal Infection: Results From a Randomized, Controlled, Double-Blind, Phase 3 Program. Clin Infect Dis. 2016;62(11):1380-1389. doi:10.1093/cid/ciw133
- Lucasti C, Hershberger E, Miller B, et al. Multicenter, double-blind, randomized, phase II trial to assess the safety and efficacy of ceftolozane-tazobactam plus metronidazole compared with meropenem in adult patients with complicated intra-abdominal infections. Antimicrob Agents Chemother. 2014;58(9):5350-5357. doi:10.1128/AAC.00049-14
- Lucasti C, Vasile L, Sandesc D, et al. Phase 2, Dose-Ranging Study of Relebactam with Imipenem-Cilastatin in Subjects with Complicated Intra-abdominal Infection. Antimicrob Agents Chemother. 2016;60(10):6234-6243. Published 2016 Sep 23. doi:10.1128/AAC.00633-16
- Motsch J, Murta de Oliveira C, Stus V, et al. RESTORE-IMI 1: A Multicenter, Randomized, Double-blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs Colistin Plus Imipenem in Patients With Imipenem-nonsusceptible Bacterial Infections. Clin Infect Dis. 2020;70(9):1799-1808. doi:10.1093/cid/ciz530
- Solomkin J, Hershberger E, Miller B, et al. Ceftolozane/Tazobactam Plus Metronidazole for Complicated Intra-abdominal Infections in an Era of Multidrug Resistance: Results From a Randomized, Double-Blind, Phase 3 Trial (ASPECT-cIAI). Clin Infect Dis. 2015;60(10):1462-1471. doi:10.1093/cid/civ097
- Another possibility would be cefiderocol as well
- Other situations:
- If a Pseudomonas isolate had been susceptible to both a non-carbapenem beta-lactam agent (such as pip-tazo, ceftazidime, cefepime) and carbapenem → preferred to use traditional beta-lactam agent or fluoroquinolone in effort to preserve activity of carbapenems for future increasingly drug-resistant infections
- Another scenario might be isolates that are non-susceptible to any carbapenem (meropenem or imipenem MICs ≥4 µg/mL) but susceptible to traditional beta-lactams → here could administer traditional agent as high dose extended infusion therapy
- This phenotype accounts for ~20-60% of carbapenem-resistant PsA
- Gill CM, Aktaş E, Alfouzan W, et al. Elevated MICs of Susceptible Antipseudomonal Cephalosporins in Non-Carbapenemase-Producing, Carbapenem-Resistant Pseudomonas aeruginosa: Implications for Dose Optimization. Antimicrob Agents Chemother. 2021;65(11):e0120421. doi:10.1128/AAC.01204-21
- Khalili Y, Yekani M, Goli HR, Memar MY. Characterization of carbapenem-resistant but cephalosporin-susceptible Pseudomonas aeruginosa. Acta Microbiol Immunol Hung. 2019;66(4):529-540. doi:10.1556/030.66.2019.036
- Zaidenstein R, Miller A, Tal-Jasper R, et al. Therapeutic Management of Pseudomonas aeruginosa Bloodstream Infection Non-Susceptible to Carbapenems but Susceptible to “Old” Cephalosporins and/or to Penicillins. Microorganisms. 2018;6(1):9. Published 2018 Jan 16. doi:10.3390/microorganisms6010009
- Zeng ZR, Wang WP, Huang M, Shi LN, Wang Y, Shao HF. Mechanisms of carbapenem resistance in cephalosporin-susceptible Pseudomonas aeruginosa in China. Diagn Microbiol Infect Dis. 2014;78(3):268-270. doi:10.1016/j.diagmicrobio.2013.11.014
- Generally due to lack of or limited production of OprD, which normally facilitates entry of carbapenem agents into PsA with or without overexpression of efflux pumps
- This phenotype accounts for ~20-60% of carbapenem-resistant PsA
- This is sometimes not typically your first option because we hope to preserve effectiveness of novel BLBLIs for future infections, but in cases of treatment of serious infections outside of the urinary tract, this is the ideal option
Is there anything to help us choose between these newer agents if most things are equal or demonstrate susceptibility?
- Always obtain AST results for DTR-Pseudomonas aeruginosa infections to guide treatment decisions
- Regional differences in susceptibility may exist
- One useful pearl regarding BLBLIs: ceftolozane and ceftazidime have similar structure – but ceftolozane is less impact by PDC hydrolysis and porin loss than ceftazidime
- Ceftolozane does not rely on an inhibitor to restore susceptibility to an otherwise inactive beta-lactam agent (ie, ceftolozane has independent activity against DTR-PsA and does not need to rely on tazobactam to maintain its activity against DTR-PsA), which may explain its slightly higher likelihood of activity against DTR-PsA compared to other novel BLBLI
- Neither ceftazidime nor imipenem are active against DTR-PsA → avibactam and relebactam expand the activity of these agents by inhibiting PDCs
- Murano K, Yamanaka T, Toda A, et al. Structural requirements for the stability of novel cephalosporins to AmpC beta-lactamase based on 3D-structure. Bioorg Med Chem. 2008;16(5):2261-2275. doi:10.1016/j.bmc.2007.11.074
- Castanheira M, Mills JC, Farrell DJ, Jones RN. Mutation-driven β-lactam resistance mechanisms among contemporary ceftazidime-nonsusceptible Pseudomonas aeruginosa isolates from U.S. hospitals. Antimicrob Agents Chemother. 2014;58(11):6844-6850. doi:10.1128/AAC.03681-14
- Also could note that vaborbactam doesn’t really expand activity of meropenem against DTR-PsA so wouldn’t use (that’s why there are no breakpoints)
- Another important branch point would be the presence of metallo-b-lactamase, which would indicate need to use cefiderocol
- Although this is rare in the US, such isolates are more common in other areas of the world
- Is see an isolate exhibiting resistant to available BLBLIs, that should raise your suspicion for MBL production
- Timsit JF, Paul M, Shields RK, et al. Cefiderocol for the Treatment of Infections Due to Metallo-B-lactamase-Producing Pathogens in the CREDIBLE-CR and APEKS-NP Phase 3 Randomized Studies. Clin Infect Dis. 2022;75(6):1081-1084. doi:10.1093/cid/ciac078
- Mushtaq S, Sadouki Z, Vickers A, Livermore DM, Woodford N. In Vitro Activity of Cefiderocol, a Siderophore Cephalosporin, against Multidrug-Resistant Gram-Negative Bacteria. Antimicrob Agents Chemother. 2020;64(12):e01582-20. Published 2020 Nov 17. doi:10.1128/AAC.01582-20
Additional points made by our discussants on management of DTR-PsA infections
- Emergence of resistance of DTR-PsA isolates to newer beta-lactam agents is a concern
- Data suggest frequency may be highest for ceftolozane-tazobactam and ceftazidime-avibactam
- Role of combination therapy – not suggested for infections with confirmed susceptibility to agents we have discussed
- Regardless of the antibiotic agent administered, patients infected with P. aeruginosa should be closely monitored to ensure clinical improvement as P. aeruginosa exhibits an impressive capacity to iteratively express additional resistance mechanisms while exposed to antibiotic therapy
- Clinicians are advised to request repeat AST of subsequent clinical MDR-P. aeruginosa isolates obtained from the same patient to monitor for the development of resistance.
What other options might be at our disposal for severe MDR/DTR-PsA infections?
- Bacteriophage therapy
- Viruses that bind to bacteria, transfer viral DNA/RNA, produce virions that lyse bacterial cell, phage progeny infect other bacterial cells
- Each bacteriophage is specific to one or a few strains of bacteria (may spare human microbiome, do not infect eukaryotic cells, reduced adverse events)
- Self-replicating and self-limited since only multiply in presence of specific bacterial host
- Dr. Efteraj also mentioned vaccine
Infographics
Goal
Listeners will be able to understand possible management strategies for treatment of MDR/DTR-Pseudomonas aeruginosa infections
Learning Objectives
After listening to this episode, listeners will be able to:
- Define multidrug resistant and difficult to treat resistance Pseudomonas aeruginosa
- Describe possible resistance mechanisms for Pseudomonas
- Compare carbapenemase production prevalence globally
- Describe available antimicrobials for DTR-Pseudomonas aeruginosa
Disclosures
Our guests as well as Febrile podcast and hosts report no relevant financial disclosures
Citation
Alshehail, B., Alhowity, E., Dong, S. “#86: WAAW with SPIDS – Deep Dive into DTR Pseudomonas”. Febrile: A Cultured Podcast. https://player.captivate.fm/episode/29722c75-69bd-41d8-888b-5044229fce59


