Credits
Hosts: Mohamed Bohlega, Sara Dong
Guest: Varun Phadke
Writing: Mohamed Bohlega, Sara Dong
Producing/Editing/Cover Art/Infographics: Sara Dong
Our Guests
Varun Phadke, MD

Varun Phadke earned his MD degree from Harvard Medical School and completed his residency training at New York-Presbyterian Hospital / Columbia University Medical Center. He then moved to Atlanta for his ID fellowship training in infectious diseases at Emory. He has been on Emory faculty since that time and serves as the Associate Program Director for the ID Fellowship Program. He is also a core faculty member in the internal medicine residency program at Emory. He previously completed a fellowship in diagnostic excellence through the Society to Improve Diagnosis in Medicine and is passionate about teaching clinical reasoning. He also serves as Chair of the Teaching and Learning Resources Work Group of the IDSA Medical Education Community of Practice and Chair of Education Workgroup of the American Society of Transplantation ID Community of Practice.
Mohamed Bohlega, MD

Mohamed Bohlega is a PGY-3 internal medicine resident at The George Washington University. He is passionate about all things related to infectious disease medical education and will be an ID fellow at Washington University
Culture
Varun has been enjoying the newest season of the Great British Bake-Off and completed the series Queens Gambit (on Netflix)
Consult Notes
Consult Q
Patient was found to have vegetations on echocardiography, please assist with evaluation and antimicrobial management
One-liner
36 year-old male with HIV and ESRD on PD with culture negative endocarditis
Key Points
Jump to:
Varun started by taking a moment to slow down and identify the key aspects of the case with problem representation! Here’s a throwback to a prior graphic from an early episode with Gerome Escota.
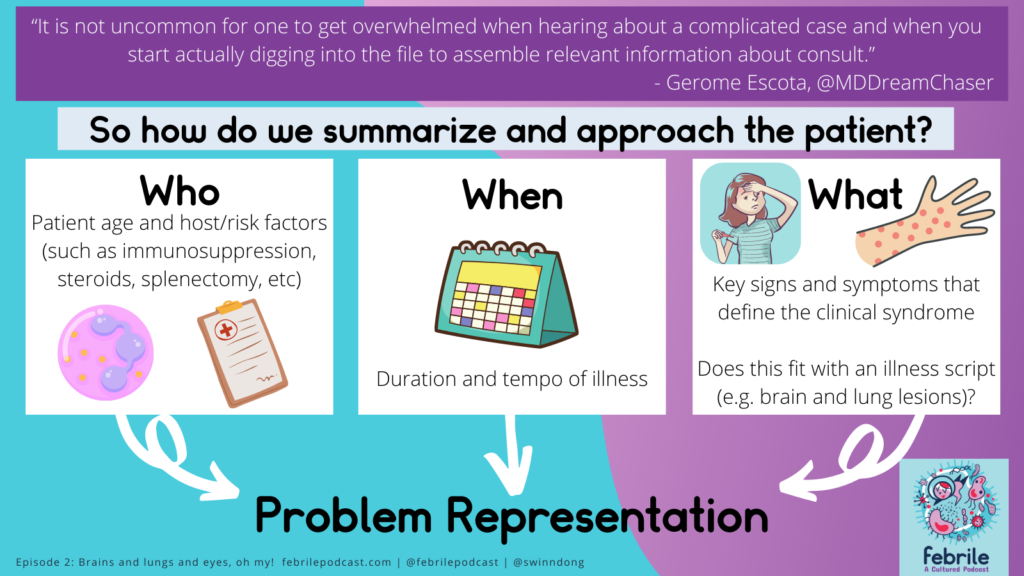
As we get started, it’s important to think about the syndrome or diagnosis of concern here — infective endocarditis (IE)!
- The diagnosis of endocarditis is based on clinical manifestations, blood cultures (or other micro data), and echocardiography findings
- The accepted criteria for diagnosis of infective endocarditis are the modified Duke Criteria, which are summarized in one of this episode’s graphics and below

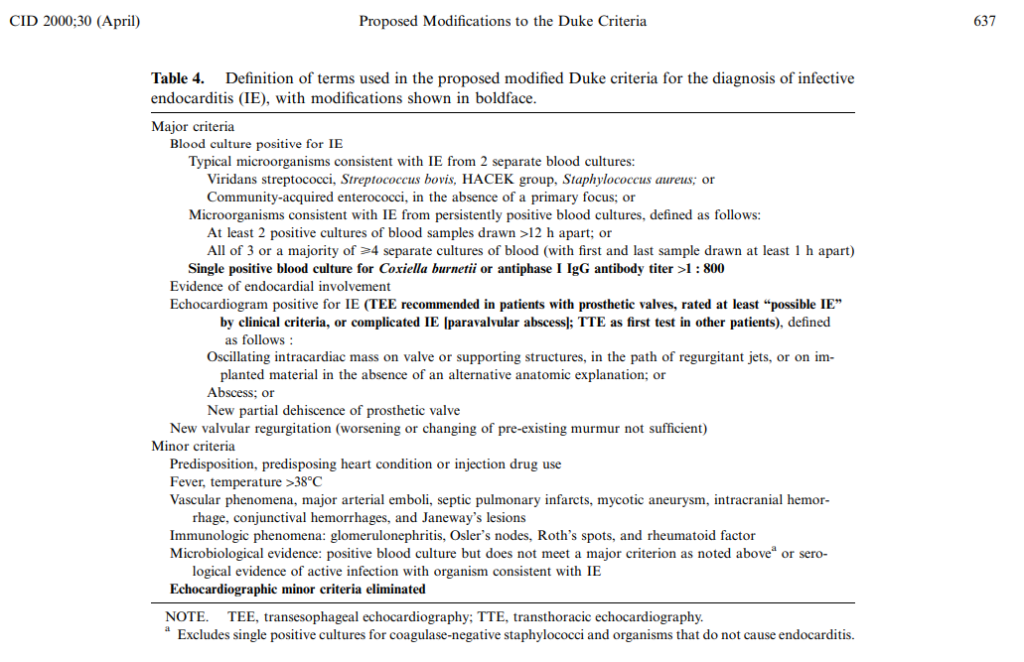
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633-638. doi:10.1086/313753
- Here is a recent NEJM review of native valve infective endocarditis that also covers culture negative (check out Table 2): Chambers HF, Bayer AS. Native-Valve Infective Endocarditis. N Engl J Med. 2020;383(6):567-576. doi:10.1056/NEJMcp2000400
Defining culture negative infective endocarditis (CNIE)
How do we define culture negative infective endocarditis?
No etiology found after 3 independent/separate blood cultures remain negative for at least 7 days incubation
How often does CNIE occur?
- Many studies placed the frequency of CNIE somewhere between 10-15% of endocarditis cases, but this proportion has been reported higher (20-30%) in European studies. As we discussed in the show, the proportion of endocarditis that is culture negative is going to vary among countries and different centers.
- Differences in frequency likely reflect the incidence of fastidious zoonotic organisms (eg, Coxiella or Brucella) that cause infection in various locations, the differences in microbiologic techniques, or availability of antibiotics without prescription
- For example, older literature on CNIE partially had higher proportions of culture negative endocarditis that was shaped by diagnostic tools available to identify etiologies of endocarditis (such as previous sensitivity of blood culture methods).
Some references and frequently cited resources
- Berbari EF, Cockerill FR 3rd, Steckelberg JM. Infective endocarditis due to unusual or fastidious microorganisms. Mayo Clin Proc. 1997;72(6):532-542. doi:10.4065/72.6.532
- Hoen B, Selton-Suty C, Lacassin F, et al. Infective endocarditis in patients with negative blood cultures: analysis of 88 cases from a one-year nationwide survey in France. Clin Infect Dis. 1995;20(3):501-506. doi:10.1093/clinids/20.3.501
- Pazin GJ, Saul S, Thompson ME. Blood culture positivity: suppression by outpatient antibiotic therapy in patients with bacterial endocarditis. Arch Intern Med. 1982;142(2):263-268.
- Werner M, Andersson R, Olaison L, Hogevik H. A clinical study of culture-negative endocarditis. Medicine (Baltimore). 2003;82(4):263-273. doi:10.1097/01.md.0000085056.63483.d2
- Brouqui P, Raoult D. Endocarditis due to rare and fastidious bacteria. Clin Microbiol Rev. 2001;14(1):177-207. doi:10.1128/CMR.14.1.177-207.2001
- Houpikian P, Raoult D. Diagnostic methods. Current best practices and guidelines for identification of difficult-to-culture pathogens in infective endocarditis. Cardiol Clin. 2003;21(2):207-217. doi:10.1016/s0733-8651(03)00028-6
- Lamas CC, Fournier PE, Zappa M, et al. Diagnosis of blood culture-negative endocarditis and clinical comparison between blood culture-negative and blood culture-positive cases. Infection. 2016;44(4):459-466. doi:10.1007/s15010-015-0863-x
What are the causes of culture negative infective endocarditis? Varun shared his framework to approach the differential diagnosis and subsequent work-up you’d recommend for CNIE. This is related to “true” culture negative infective endocarditis, but you must also consider nonbacterial endocarditis as well.
Bucket 1: “Should’ve been culture positive” aka preceding antibiotic
- These are typical endocarditis organisms, such as Staph, Strep, Enterococcus, HACEK, and so on
- Previous administration of antimicrobials can reduce recovery rate of bacteria by 35-40%! Even just one dose might sterilize cultures and wipe out your organism, especially typical Strep spp
- You often have to be quite meticulous in your history and data gathering to find this out: recent outpatient visits with antibiotic prescriptions? Recent hospitalizations or procedures? Checking pharmacy dispense records or dialysis unit dispensed meds?
Bucket 2: “Could be culture positive, if you try really hard”
- Bartonella (henselae and quintana), Brucella spp, anaerobic bacteria (such as C.acnes which needs extended incubation), Legionella spp, Chlamydia spp, mycobacteria (such as M.chimaera in post-cardiac surgery pt exposed to contaminated heater-cooler units), fungal (Cryptococcus, Histoplasma)
- Highly fastidious bacteria or nonbacterial pathogens (organism may be slow growing, require special media or culture techniques etc) → commonly we have alternative tests such as antigen, serology or PCR
- These often have unique epidemiologic risk factors or specific history clues (such as cardiac surgery → M.chimaera)
Bucket 3: “Will never be culture positive”
- Coxiella burnetti, T.whipplei, Aspergillus
- These also often have unique epi associations or might occur specifically in immunocompromised hosts
A few notes:
- As we’ve discussed, etiology will vary with geographic and epidemiological risk factors
- Two important organisms that are often heavily represented in the literature on CNIE = Coxiella, Bartonella
- **For bucket #1** Although HACEK organisms (Haemophilus spp, Aggregatibacter spp, Cardiobacterium hominis, Eikenella corrodens, Kingella spp) and nutritionally variant Strep spp (Granulicatella, Abiotrophia) were classically listed under CNIE, these bacteria should grow and be identified with modern culture methods!!!!
- For resources on epidemiologic associations, there is a list in UpToDate as well as the AHA endocarditis guidelines in Table 6
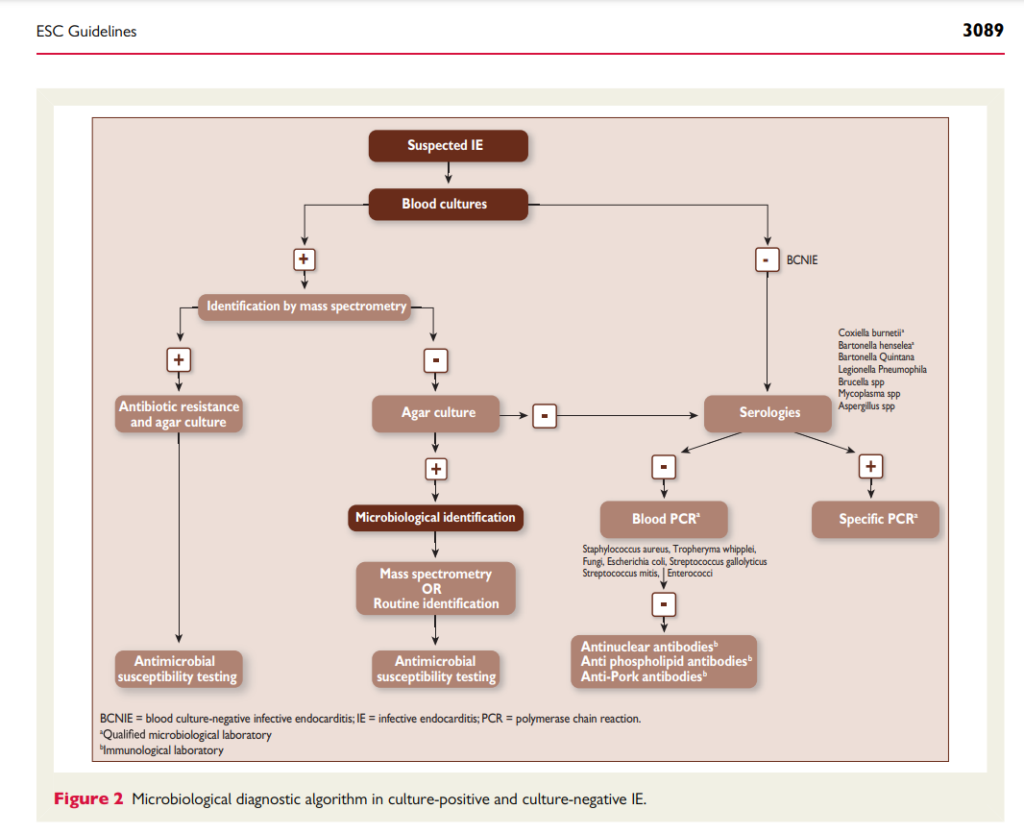
From an ID standpoint, the work-up for culture negative endocarditis can be a huge challenge as we try to work through which diagnostics to send. Varun discussed his approach to testing and I’ve summarized some notes below.
- In addition to your routine blood cultures, there are additional tests from bloodwork that can be sent. These will vary depending on local and patient-specific epidemiologic risk factors, but:
- Serology for zoonotic agents, in particular:
- Bartonella serology
- Coxiella serology (phase 1 serology now a major criteria in the modified Duke criteria)
- Brucella Ab
- Extended cultures as appropriate such as:
- Brucella cultures
- AFB blood cultures, often if immunocompromised host or concern for M.chimaera
- Fungal blood cultures if considering endemic fungi such as Histoplasma (remember that Candida and Cryptococcus will grow in routine blood cultures)
- Fungal antigen and/or serology testing
- In settings with concern for nonbacterial endocarditis, might consider assays for antinuclear antibodies, rheumatoid factor, antiphospholipid syndrome
- Serology for zoonotic agents, in particular:
- For patients that go for surgery, their excised valve tissue can be sent for testing as noted below. It is important to be deliberate about requesting tests beforehand so tissue is sent to the right places!
- Bacterial, fungal, and AFB cultures
- Histopathology with:
- Special stains (such as Warthin-Starry for Bartonella)
- Immunohistochemistry (for Coxiella, Bartonella, T.whipplei)
- Additional techniques include:
- Organism specific PCR assays
- In particular for Bartonella as Varun mentioned, which will have better performance for this organism compared to universal PCR
- 16S (bacteria) and 18S (fungi) ribosomal RNA PCR (aka universal PCR) from valvular tissue
- You should perform this test as well as organism-specific PCRs noted above from valvular vegetation or abscess material rather than blood for higher sensitivity!!
- Metagenomic next generation sequencing
- Not a very robust evidence base for routine use of this testing, and there is probably some publication bias for positive results — but might be a useful tool in certain patients
- Organism specific PCR assays
- Check out these two overviews as you think about testing:
- In a large prospective analysis of samples sent to reference lab for CNIE:
- Pathogen was identified in 63% of 759 cases
- Serologic results were positive in 48% (primarily for Coxiella and Bartonella)
- PCR identified pathogen in ⅔ valves studied
- No cause identified in 35% cases
- Fournier PE, Thuny F, Richet H, et al. Comprehensive diagnostic strategy for blood culture-negative endocarditis: a prospective study of 819 new cases. Clin Infect Dis. 2010;51(2):131-140. doi:10.1086/653675
- In a follow-up study, the addition of RT-PCR assays were added.
- Etiology was found in 78% of cases
- Check out the distribution of pathogens by diagnostic method used
- Fournier PE, Gouriet F, Casalta JP, et al. Blood culture-negative endocarditis: Improving the diagnostic yield using new diagnostic tools. Medicine (Baltimore). 2017;96(47):e8392. doi:10.1097/MD.0000000000008392
- In a large prospective analysis of samples sent to reference lab for CNIE:
- A few other references:
- Raoult D, Casalta JP, Richet H, et al. Contribution of systematic serological testing in diagnosis of infective endocarditis. J Clin Microbiol. 2005;43(10):5238-5242. doi:10.1128/JCM.43.10.5238-5242.2005
- Godfrey R, Curtis S, Schilling WH, James PR. Blood culture negative endocarditis in the modern era of 16S rRNA sequencing. Clin Med (Lond). 2020;20(4):412-416. doi:10.7861/clinmed.2019-0342
- Subedi S, Jennings Z, Chen SC. Laboratory Approach to the Diagnosis of Culture-Negative Infective Endocarditis. Heart Lung Circ. 2017;26(8):763-771. doi:10.1016/j.hlc.2017.02.009
- Liesman RM, Pritt BS, Maleszewski JJ, Patel R. Laboratory Diagnosis of Infective Endocarditis. J Clin Microbiol. 2017;55(9):2599-2608. doi:10.1128/JCM.00635-17
- Han D, Li R, Shi J, Tan P, Zhang R, Li J. Liquid biopsy for infectious diseases: a focus on microbial cell-free DNA sequencing. Theranostics. 2020;10(12):5501-5513. Published 2020 Apr 7. doi:10.7150/thno.45554
- Kolb M, Lazarevic V, Emonet S, et al. Next-Generation Sequencing for the Diagnosis of Challenging Culture-Negative Endocarditis. Front Med (Lausanne). 2019;6:203. Published 2019 Sep 20. doi:10.3389/fmed.2019.00203
- Maneg D, Sponsel J, Müller I, et al. Advantages and Limitations of Direct PCR Amplification of Bacterial 16S-rDNA from Resected Heart Tissue or Swabs Followed by Direct Sequencing for Diagnosing Infective Endocarditis: A Retrospective Analysis in the Routine Clinical Setting. Biomed Res Int. 2016;2016:7923874. doi:10.1155/2016/7923874
Will end with an overview of management of antibiotics in CNIE. As Varun discussed, antibiotic decision making ultimately comes down to balancing risks and benefits.
The guidelines generally recommend two agents (such as vancomycin with ceftriaxone or amp-sulbactam) that cover bucket #1 (should’ve been culture positive) because:
- The base rate and likelihood of these typical organisms is high
- The risk of toxicity are well known (we use these drug commonly and empirically)
In addition, you can add doxycycline as well to help target Bartonella or other atypical organisms. See more details about what is written out in AHA and ESC guidelines below
- For patients with acute clinical presentation (symptoms for days), antimicrobial therapy for S.aureus, beta-hemolytic streptococci, and aerobic gram negative bacilli is reasonable → vancomycin + cefepime
- For patients with subacute presentation (symptoms for weeks), antimicrobial therapy for S.aureus, viridans group streptococci, HACEK organisms, Enterococci is reasonable → vancomycin + ampicillin-sulbactam
- Can modify depending on clinical circumstances such as
- If GPC or HACEK most likely cause, sometimes use combo of vanc/ceftriaxone
- If on hemodialysis, could consider vancomycin alone
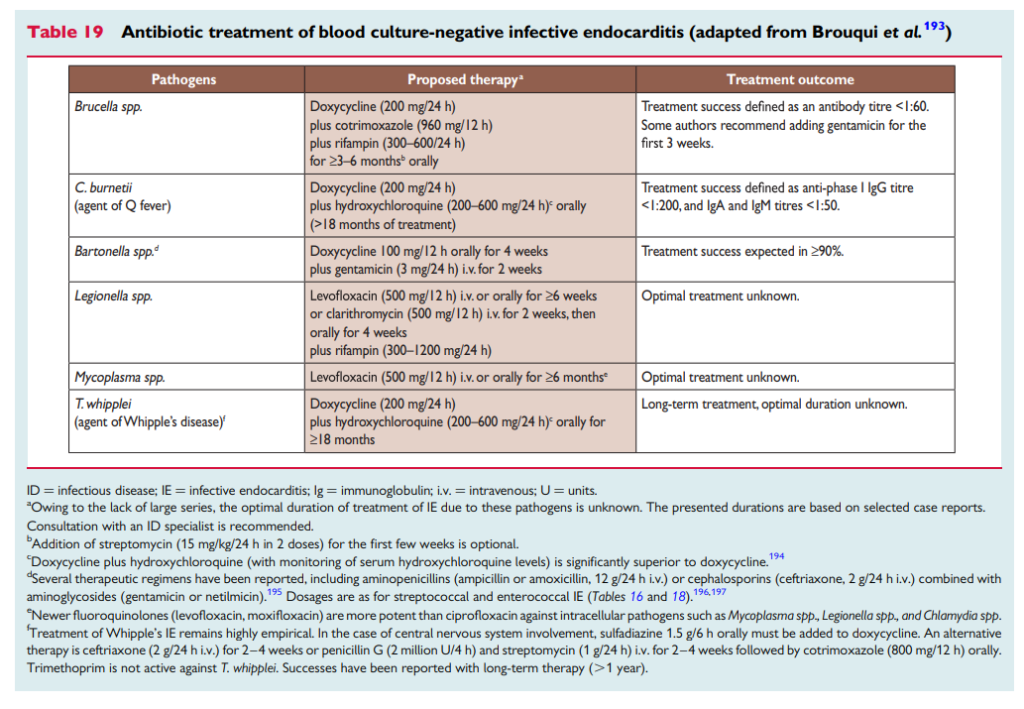
But what about mycobacterial and fungal etiologies? Varun explained the ongoing balance of risk and benefits that influence this decision. Antimycobacterial agents such as rifampin or antifungal therapy generally are only used if we have compelling data, high pretest probability or diagnostic confidence because the risk of drug toxicity and interactions is much higher with these agents
The duration of 6 wks is often used because of the typical organisms in bucket #1, but there is not often a clear answer here (including from the guidelines). This is substantially shorter than durations recommended for Coxiella or Brucella treatment (which are months) — but again, committing to that duration of therapy leads to a place where risks outweigh benefits. In addition, the duration of these prolonged treatments are often based on following antibody titers — which we can’t use to stop therapy when the baseline Ab is negative. The main diagnostic test that is useful is TIME! You often will treat your patient for 6 wks and then monitor for symptoms after discontinuation of therapy.
Take-home advice from Varun: it’s important to always be critical of the data we are gathering:
- History: are we sure there was no antecedent antibiotic exposure?
- Echo: are we sure that this finding is new compared to prior echos?
- Lab tests: is this negative result enough to rule out Bartonella
Approach every piece of data with that healthy skepticism and it will make you a more precise data gatherer and interpreter — and often help make those challenging diagnoses!
Episode Art & Infographics
Goal
Listeners will be able to approach the differential diagnosis and work-up for culture negative infective endocarditis.
Learning Objectives
After listening to this episode, listeners will be able to:
- Define culture negative infective endocarditis (CNIE)
- List the possible organisms that cause CNIE
- Describe the available diagnostics for evaluation for CNIE
Disclosures
Our guest (Varun Phadke) as well as Febrile podcast and hosts report no relevant financial disclosures
Citation
Phadke, V., Bohlega, M., Dong, S. “#26: How to Develop Your Culture Negatives”. Febrile: A Cultured Podcast. https://player.captivate.fm/episode/95c4bf3c-3869-4502-b05a-b53a0fa19356


