Credits
Host(s): Sara Dong
Guest: Camille Kotton
Writing/Producing/Editing/Cover Art/Infographics: Sara Dong
Camille Nelson Kotton MD, FIDSA, FAST is the clinical director of the Transplant Infectious Disease and Immunocompromised Host Program at the Massachusetts General Hospital, and Associate Professor of Medicine, Harvard Medical School, Boston, Massachusetts. She was chair of The Infectious Disease Community of Practice of The American Society of Transplantation (2012-2018). From 2007-2013, she was the president of The Transplant Infectious Disease Section of The Transplantation Society. Highlights of her time as president include the development of international guidelines on CMV management after solid organ transplant, published in Transplantation (2010, 2013, 2018). She is the first transplant infectious disease specialist to be a councilor of The Transplantation Society (2020). Dr. Kotton has authored the past three versions of the American Society of Transplantation Travel Medicine guidelines, and has been an author of the CDC Yellow Book Chapter on Immunocompromised Hosts and Travel for the past decade. Her clinical interests include vaccinations in transplant candidates and recipients, cytomegalovirus, zoonoses, and travel and tropical medicine in the transplant setting. She is a member of the CDC Advisory Committee on Immunization Practices (ACIP) and is involved in national decisions regarding COVID-19 vaccines.
Culture
Camille has been enjoying music again recently, especially while riding Peloton. She specifically mentioned having fun listening and singing along to Jackson Browne in the car on the way to work!
Consult Notes
Consult Q
70 year-old male who is status post renal transplant presents with abdominal pain and diarrhea. Please evaluate for infectious causes in this immunocompromised host.
Case Summary
70 year old male s/p DDRT (CMV D+/R-) who presented with resistant CMV colitis with UL 97 mutation
Key Points

Don’t miss
Part 1 of the Troll of Transplantation with Drs. Kevin He and Joseph Sassine: https://febrilepodcast.com/33-troll-transplant-1/
Let’s start with some key resources again!
What is the anticipated timeframe for a response to antiviral therapy in a CMV case in transplant recipient?
- One-log decline in CMV viral load is anticipated after at least 2 weeks of appropriately dosed antiviral therapy >> so this is a reminder that the viral load may be the same or higher on the first repeat test after starting therapy
- Camille’s tip mentioned on the show is remembering the difference between the terms viremia and DNAemia, which will help understand why the numbers don’t come down immediately. For DNAemia, we’re just measuring the amount of CMV DNA in the bloodstream, which doesn’t reflect or differentiate between live or dead virus. You can think that after starting treatment, you likely lysed a bunch of cells, and maybe there’s a lot of dead CMV circulating.
What are the potential risk factors/reasons that ID fellows and trainees need to be thinking about when weighing whether someone is at risk for refractory/resistant CMV infection?
- Host factors
- D+/R- serostatus
- Potent immunosuppression / Over-immunosuppressed status (absence or deficiency in CMV-specific T-cell immunity)
- Previous prolonged CMV antiviral exposure (>3 months)
- Recurrent CMV infection
- Prolonged subtherapeutic antiviral drug concentrations (low dose, poor absorption) >> as emphasized on the show – dosing antivirals appropriately is crucial!!
- Viral factors
- High viral load (VL) at start of therapy
- Ongoing low level CMV viremia
- Failure of CMV VL to fall despite appropriate therapy
- Rise in CMV VL after decline on appropriate therapy
- Resistance to GCV or other antiviral drugs
How do we test for suspected drug-resistant CMV? What are we actually testing for here?
We are obtaining resistance testing by testing for specific gene mutations. Genotypic resistance assays are preferred over culture-based phenotypic assays >> and the tests are performed on viral sequences directly amplified from blood (whole blood, plasma, leukocytes), body fluids (urine, CSF, BAL, vitreous), tissue
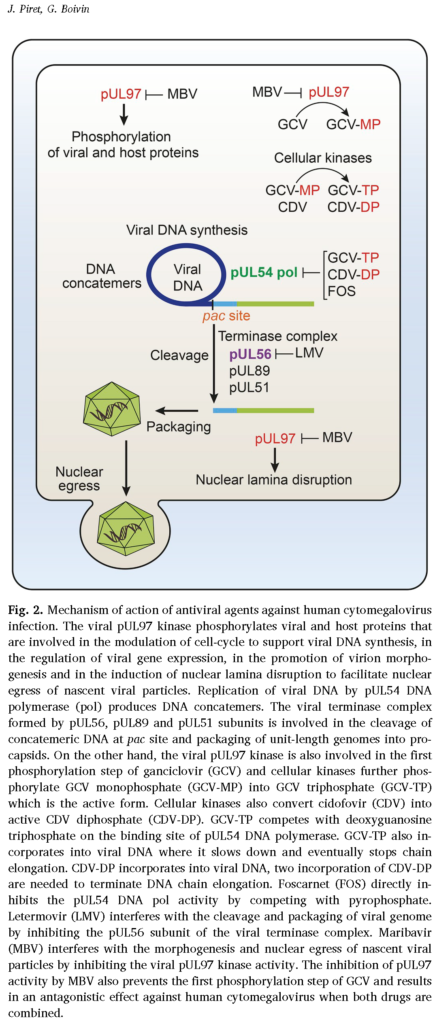
- I like this figure to the left for an overview of the mechanism of action for CMV meds (from: Piret J, Boivin G. Clinical development of letermovir and maribavir: Overview of human cytomegalovirus drug resistance. Antiviral Res. 2019;163:91-105. doi:10.1016/j.antiviral.2019.01.011)
- UL97: encodes a viral kinase that catalyzes initial mono-phosphorylation and activation of ganciclovir (GCV)
- Subsequent phosphorylation by cellular kinases leads to active GCV-triphosphate (nucleoside analog), which serves as competitive substrate for incorporation into elongating CMV DNA, a process catalyzed by CMV DNA polymerase (an enzyme encoded by UL54)
- Since foscarnet (FOS) and cidofovir (CDV) also inhibit UL54-encoded CMV DNA polymerase, mutations may confer cross-resistance to GCV, FOS, CDV
- Letermovir mutations correlate with UL56 (less commonly also UL51 and UL89, which encode viral terminase complex)
- UL97 kinase gene mutations appear first in about 90% of cases >> UL54 mutations usually evolve later
- Test results will report the mutations out as a list usually, but other details are not always included. As Camille mentioned throughout the episode, the degree of resistance to GCV conferred by UL97 depends on site of mutation
- Most common high level resistance genetic mutations: M460V/I, H520Q, C592G, A594V, L595S, and C603W
- You can see a CMV UL54 gene mutation map in the International Guidelines Figure 1 as well as GCV resistance levels associated with selected UL97 genotypes in Table 8
- Camille mentioned referring to Dr. Sunwen Chou’s papers for reference and to identify relative levels of drug resistance of mutations. Here are a few to save!
- A review of drug resistance: Hakki M, Chou S. The biology of cytomegalovirus drug resistance. Curr Opin Infect Dis. 2011;24(6):605-611. doi:10.1097/QCO.0b013e32834cfb58
- Chou S. Advances in the genotypic diagnosis of cytomegalovirus antiviral drug resistance. Antiviral Res. 2020;176:104711. doi:10.1016/j.antiviral.2020.104711
- Chou S. Approach to drug-resistant cytomegalovirus in transplant recipients. Curr Opin Infect Dis. 2015;28(4):293-299. doi:10.1097/QCO.0000000000000170
What are our treatment options for resistant CMV infections? Our treatment options are limited!
- First: reduce immunosuppression as much as able (but an approach to this is not defined or standardized currently)
- There are no controlled trials to guide the optimal choice of antiviral treatment of resistant CMV infection.
- Ganciclovir (GCV)-resistant CMV isolates with UL97 only are usually susceptible to foscarnet (FOS) and cidofovir (CDV)
- Based on observational studies, FOS is first line for UL97 mutant GCV-R CMV with higher level resistance
- Some UL97 mutations though confer lower levels of GCV resistance by themselves and may be amenable to GCV dose escalation (up to 10 mg/kg every 12 hrs)
- UL54 mutations may confer mono or cross resistance to GCV, FOS, CDV, depending on the site of mutation >> More likely to be cross resistant to CDV if UL54, so FOS is generally the empiric choice
- Definitive antiviral choices should be guided by genotypic assay results
- Foscarnet salvage therapy is often successful, but the key difficulties are related to metabolic and renal toxicity (which may impair treatment outcome)
- Cidofovir has not been well studied as salvage therapy, but can be considered when GCV and FOS dual resistance is detected
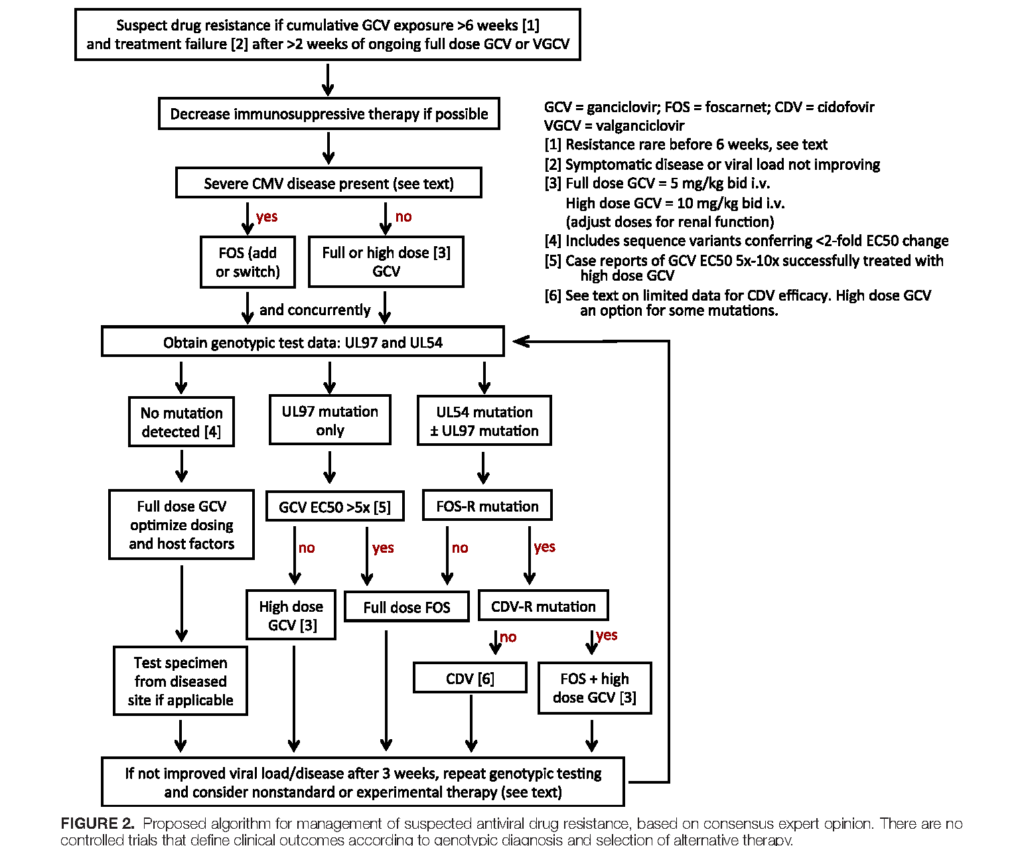
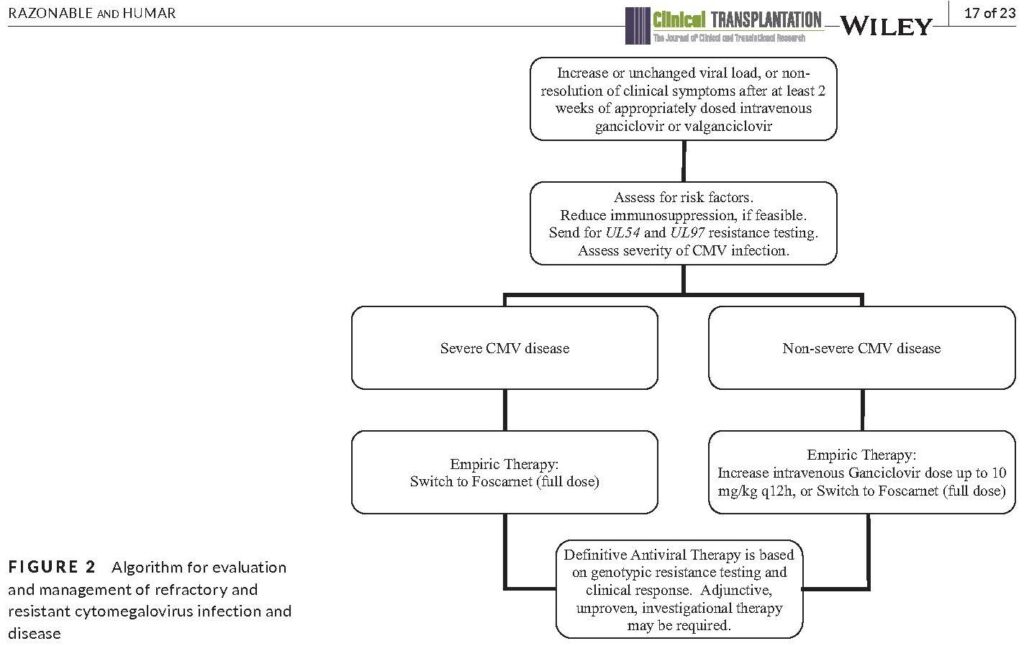
- Check out the updated algorithm from the guidelines, which are based on consensus expert opinion (reproduced below)
- International Guidelines Figure 2
- AST ID COP Guidelines Figure 2
- Maribavir is a newer CMV agent that was recently FDA approved for resistant/refractory CMV.
- This is an oral inhibitor of the CMV UL97 kinase
- Here is the phase 3 trial publication (SOLSTICE): Avery RK, Alain S, Alexander BD, et al. Maribavir for Refractory Cytomegalovirus Infections With or Without Resistance Post-Transplant: Results from a Phase 3 Randomized Clinical Trial [published online ahead of print, 2021 Dec 2]. Clin Infect Dis. 2021;ciab988. doi:10.1093/cid/ciab988
- Visual abstract reproduced below

- Investigational or newer drugs to also mention
- Letermovir (approved for prophylaxis in alloHSCT, has been used off-label for SOT). Remember that the resistance barrier to letermovir is very low! Can take just a few replication cycles to become resistant
- Brincidofovir: oral CDV derivative. Phase 3 trial in HSCT failed primarily due to severe GI toxicity >> medication is not currently available
Are there any possible adjunctive therapies?
- IVIg has been used anecdotally to supplement antiviral therapy
- This is usually in patients with hypogammaglobulinemia >> can replace with IVIg or CMV Ig (which is just enhanced IVIg from CMV+ donor pool)
- Adoptive transfer of CMV-specific T cells, derived from autologous and allogeneic sources has been used experimentally in HSCT
- Other drugs have been considered given anti-CMV effects in vitro: sirolimus and mTOR inhibitors, leflunomide
We talked with Camille about the use of immune monitoring as an emerging clinical tool to assist with CMV risk stratification and management after solid organ transplant. We focused on CMV-specific tools in the episode, but let’s start with a few notes on nonspecific immune monitoring as well.
Nonspecific measurements such as absolute lymphocyte count (ALC) and CD4 T-cell count have been correlated with risk of CMV disease after SOT >> AST ID COP guidelines suggest that ALC and CD4/CD8 T cell subsets may be used to stratify the risk of CMV disease after SOT, but specific thresholds still need to be clinically validated
- Lymphopenia is commonly used in clinical practice as a risk factor for CMV infection. Check out this paper looking at ALC: Meesing A, Razonable RR. Absolute Lymphocyte Count Thresholds: A Simple, Readily Available Tool to Predict the Risk of Cytomegalovirus Infection After Transplantation. Open Forum Infect Dis. 2018;5(10):ofy230. Published 2018 Sep 11. doi:10.1093/ofid/ofy230
- A few others:
- Meesing A, Abraham RS, Razonable RR. Clinical Correlation of Cytomegalovirus Infection With CMV-specific CD8+ T-cell Immune Competence Score and Lymphocyte Subsets in Solid Organ Transplant Recipients. Transplantation. 2019;103(4):832-838. doi:10.1097/TP.0000000000002396
- Manuel O, Husain S, Kumar D, et al. Assessment of cytomegalovirus-specific cell-mediated immunity for the prediction of cytomegalovirus disease in high-risk solid-organ transplant recipients: a multicenter cohort study. Clin Infect Dis. 2013;56(6):817-824. doi:10.1093/cid/cis993
- A few others:
- Hypogammaglobulinemia is also associated with CMV disease >> measurement of total IgG levels may be used to assess risk (weak, low in AST IC COP guidelines). Check out some of the available references in various organs below:
- Goldfarb NS, Avery RK, Goormastic M, et al. Hypogammaglobulinemia in lung transplant recipients. Transplantation. 2001;71(2):242-246. doi:10.1097/00007890-200101270-00013
- Fernández-Ruiz M, López-Medrano F, Varela-Peña P, et al. Monitoring of immunoglobulin levels identifies kidney transplant recipients at high risk of infection. Am J Transplant. 2012;12(10):2763-2773. doi:10.1111/j.1600-6143.2012.04192.x
- Doron S, Ruthazer R, Werner BG, Rabson A, Snydman DR. Hypogammaglobulinemia in liver transplant recipients: incidence, timing, risk factors, and outcomes. Transplantation. 2006;81(5):697-703. doi:10.1097/01.tp.0000180531.66518.9e
- Sarmiento E, Navarro J, Fernandez-Yañez J, Palomo J, Muñoz P, Carbone J. Evaluation of an immunological score to assess the risk of severe infection in heart recipients. Transpl Infect Dis. 2014;16(5):802-812. doi:10.1111/tid.12284
- Sarmiento E, Jaramillo M, Calahorra L, et al. Evaluation of humoral immunity profiles to identify heart recipients at risk for development of severe infections: A multicenter prospective study. J Heart Lung Transplant. 2017;36(5):529-539. doi:10.1016/j.healun.2016.10.004
- There are also nonpathogen-specific immune function assays, but there are not studies indicating whether these are predictive of CMV DNAemia or disease.
- One example is the commercially available ImmunKnow (Viracor-Eurofins) test: not specific for CMV. Looks at overall immune function and serves as marker of immunosuppression by determining the amount of ATP produced by CD4 T cells in response to whole blood stimulation.
- Another is the QuantiFERON Monitor, another global immune function test
Now let’s transition to CMV-specific immune monitoring assays. The majority of these assays rely on detection of IFN-gamma after stimulation of whole blood or peripheral blood mononuclear cells (PBMC) with CMV specific antigens or peptides >> similar to use with Quantiferon/TSPOT in tuberculosis
- Both the AST ID COP guidelines and International Consensus Guidelines have sections on this you should check out! In particular, Tables 2 and 3 from the International Guidelines in particular outline the advantages, limitations, and potential clinical uses of various assays.
- Expert consensus feels that the ideal assay should provide both quantitative and functional information about CMV specific CD4 and CD8 cells
- QuantiFERON-CMV: ELISA-based IFN-g release assay detecting CD8 T cells after peptide stimulation
- Camille discussed that this is not commercially available, but used in research settings
- Looks promising in CMV R+ patients to determine length of prophylaxis or risk of recurrent disease after end of treatment – but as Camille points out, we often are not quite as worried about those seropositive patients to begin with compared to those at higher risk (D+/R-, which it hasn’t really shown to be helpful yet in this population)
- Here are a few studies:
- Walker S, Fazou C, Crough T, et al. Ex vivo monitoring of human cytomegalovirus-specific CD8+ T-cell responses using QuantiFERON-CMV. Transpl Infect Dis. 2007;9(2):165-170. doi:10.1111/j.1399-3062.2006.00199.x
- Westall GP, Mifsud NA, Kotsimbos T. Linking CMV serostatus to episodes of CMV reactivation following lung transplantation by measuring CMV-specific CD8+ T-cell immunity. Am J Transplant. 2008;8(8):1749-1754. doi:10.1111/j.1600-6143.2008.02294.x
- Manuel O, Husain S, Kumar D, et al. Assessment of cytomegalovirus-specific cell-mediated immunity for the prediction of cytomegalovirus disease in high-risk solid-organ transplant recipients: a multicenter cohort study. Clin Infect Dis. 2013;56(6):817-824. doi:10.1093/cid/cis993
- Kumar D, Chernenko S, Moussa G, et al. Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am J Transplant. 2009;9(5):1214-1222. doi:10.1111/j.1600-6143.2009.02618.x
- Kumar D, Mian M, Singer L, Humar A. An Interventional Study Using Cell-Mediated Immunity to Personalize Therapy for Cytomegalovirus Infection After Transplantation. Am J Transplant. 2017;17(9):2468-2473. doi:10.1111/ajt.14347
- Another is the ELISpot assay (quantifies CD4 and CD8 T cells producing IFN-g in response to CMV), which has been evaluated in various settings
- International multicenter study looking at ELISPOT-based/T-SPOT.CMV: Kumar D, Chin-Hong P, Kayler L, et al. A prospective multicenter observational study of cell-mediated immunity as a predictor for cytomegalovirus infection in kidney transplant recipients. Am J Transplant. 2019;19(9):2505-2516. doi:10.1111/ajt.15315
- Chanouzas D, Small A, Borrows R, Ball S. Assessment of the T-SPOT.CMV interferon-γ release assay in renal transplant recipients: A single center cohort study. PLoS One. 2018;13(3):e0193968. Published 2018 Mar 20. doi:10.1371/journal.pone.0193968
- Gliga S, Korth J, Krawczyk A, et al. T-Track-CMV and QuantiFERON-CMV assays for prediction of protection from CMV reactivation in kidney transplant recipients. J Clin Virol. 2018;105:91-96. doi:10.1016/j.jcv.2018.06.009
- Banas B, Steubl D, Renders L, et al. Clinical validation of a novel enzyme-linked immunosorbent spot assay-based in vitro diagnostic assay to monitor cytomegalovirus-specific cell-mediated immunity in kidney transplant recipients: a multicenter, longitudinal, prospective, observational study. Transpl Int. 2018;31(4):436-450. doi:10.1111/tri.13110
- Bestard O, Lucia M, Crespo E, et al. Pretransplant immediately early-1-specific T cell responses provide protection for CMV infection after kidney transplantation. Am J Transplant. 2013;13(7):1793-1805. doi:10.1111/ajt.12256
- Abate D, Fiscon M, Saldan A, et al. Human cytomegalovirus-specific T-cell immune reconstitution in preemptively treated heart transplant recipients identifies subjects at critical risk for infection. J Clin Microbiol. 2012;50(6):1974-1980. doi:10.1128/JCM.06406-11
- Abate D, Saldan A, Fiscon M, et al. Evaluation of cytomegalovirus (CMV)-specific T cell immune reconstitution revealed that baseline antiviral immunity, prophylaxis, or preemptive therapy but not antithymocyte globulin treatment contribute to CMV-specific T cell reconstitution in kidney transplant recipients. J Infect Dis. 2010;202(4):585-594. doi:10.1086/654931
- A few that we didn’t discuss on the show include:
- Use of intracellular cytokine staining (ICS) for IFN-g using flow cytometry, which gives quantitative and qualitative characterization
- Mihm J, Leyking S, Dirks J, et al. Immune-based guidance of foscarnet treatment duration in a transplant recipient with ganciclovir-resistant cytomegalovirus infection. J Clin Virol. 2016;82:5-8. doi:10.1016/j.jcv.2016.06.013
- Sester M, Sester U, Gärtner B, et al. Levels of virus-specific CD4 T cells correlate with cytomegalovirus control and predict virus-induced disease after renal transplantation. Transplantation. 2001;71(9):1287-1294. doi:10.1097/00007890-200105150-00018
- Major histocompatibility complex (MHC)-multimer assays, which directly stain peptide-specific T-cells
- Use of intracellular cytokine staining (ICS) for IFN-g using flow cytometry, which gives quantitative and qualitative characterization
Some overviews for CMV-CMI:
- Egli A, Humar A, Kumar D. State-of-the-art monitoring of cytomegalovirus-specific cell-mediated immunity after organ transplant: a primer for the clinician. Clin Infect Dis. 2012;55(12):1678-1689. doi:10.1093/cid/cis818
- Sester M, Leboeuf C, Schmidt T, Hirsch HH. The “ABC” of Virus-Specific T Cell Immunity in Solid Organ Transplantation. Am J Transplant. 2016;16(6):1697-1706. doi:10.1111/ajt.13684
Camille’s closing thoughts on exciting items to consider in the CMV + transplant world:
- Cautious optimism about novel approaches to CMV vaccine development in the future
- CMV specific T-cells for refractory disease
- Will there be cocktail approaches to decrease the risk of antiviral resistance development in the future? Will combinations of antivirals help specific patients or with specific cases of resistant/refractory disease?
- Call your local or national CMV friends/experts for additional help!
Episode Art & Infographics
Find all the Febrile infographics here!
Goal
Listeners will be able to approach a solid organ transplant recipient with suspected refractory or resistant CMV infection.
Learning Objectives
After listening to this episode, listeners will be able to:
- Describe the expected decline in viral load in response to appropriately dosed antiviral therapy
- Define refractory and resistant CMV
- Discuss and interpret genotypic resistance testing for CMV (specifically UL97, UL54)
- Discuss the available antivirals for treatment of resistant CMV
Disclosures
Our guest (Camille Kotton) is a consultant for Merck, Takeda, Roche Diagnostics, Hookipa, Oxford Immunotecas
Febrile podcast and hosts report no relevant financial disclosures
Citation
Kotton, C., Dong, S. “#35: Troll of Transplantation Part 2”. Febrile: A Cultured Podcast. https://player.captivate.fm/episode/f027830f-de69-4086-a671-8c47133930c7



