Credits
Hosts: Frances Ue, Sara Dong
Guest: Lou Ann Bruno-Murtha
Writing: Frances Ue, Sara Dong
Producing/Editing/Cover Art: Sara Dong
Infographics: Marcela Santana, Sara Dong
Our Guests
Lou Ann Bruno-Murtha, DO

Dr. Lou Ann Bruno Murtha is the Division Chief of Infectious Diseases, Medical Director of Infection Prevention at Cambridge Health Alliance (CHA), and Assistant Professor at Harvard Medical School. She received a DO in Medicine at the University of Medicine and Dentistry in New Jersey – School of Osteopathic Medicine, and completed Internal Medicine residency at St. Vincent’s Hospitalist and Medical Center in New York and Infectious Diseases fellowship at Boston University Medical Center. She joined the Department of Medicine faculty at CHA in 1991 and has since focused on hospital epidemiology, infection prevention, antimicrobial stewardship, and quality improvement. Dr. Bruno-Murtha is a sought after educator and provides teaching to medical students, pharmacy residents, and internal medicine residents. She has provided mentorship to quite a few residents who have gone on to careers in infectious diseases, including 2 that have returned as faculty. She is the consummate physician leader with her work showcased in various news and academic publications, including this case that was featured in Boston Magazine.
Frances Ue, MD, MPH

Dr. Frances Ue is an experienced clinician educator, researcher, and leader with a background in public health and advocacy. Her passion for working with diverse, underserved communities and advancing social justice, has culminated in a career as an Internal Medicine attending physician and core teaching faculty at Cambridge Health Alliance (CHA) in Cambridge, Massachusetts. She completed Internal Medicine training at CHA, and also served as Chief Resident, above all during the COVID-19 pandemic. She has developed countless curricula for medical students and residents, and is a sought after educator. She completed the Harvard Macy post-graduate clinician educators course , and returned as junior faculty this past year. She notably led an innovative curriculum utilizing unscripted cases for clinical reasoning called ‘CHAse files,’ and is currently evaluating its impact on learners and faculty. Her research is mainly in the areas of medical education and infectious diseases. Her writing can be found in STAT Opinion and the New England Journal of Medicine – Insights in Residency Training blog . She holds an Honors Bachelor of Science from Queen’s University in Canada, Master’s of Public Health from Columbia University in New York, and Doctorate of Medicine from Saint Louis University School of Medicine.
Marcela Araújo de Oliveira Santana

I am a medical student at Federal University of Uberlândia, completing my final year (6th year). I was born and raised in a small village in Minas Gerais, Brazil. I am hoping to be an internal medicine resident soon with the intent of specializing in infectious diseases! My interests are medical education, infectious diseases (especially tropical diseases), and rural health! I love reading (I am a big fan of dystopias), cooking, and playing board games with my friends.
Culture
Lou Ann is working on mastering the game of golf and her short game!
Consult Notes
Consult Q
Assistance with work-up of unremitting fever
One-liner
Elderly male who presented with fever, myalgias, and weakness and was found to have severe leptospirosis (L.interrogans, icterohemorrhagiae (serogroup). His case was consistent with Weil’s disease given hyperbilirubinemia and acute renal failure!
Key Points
Jump to Leptospirosis topics:
Before we get to leptospirosis, a few quick links on tularemia. Tularemia is often on the differential diagnosis, especially when considering animal contact
- Here is a tweetorial on tularemia I previously wrote: https://twitter.com/BIDMC_IDFellows/status/1297876128363941890
- Here is a paper from an outbreak of primary pneumonic tularemia on Martha’s Vineyard, where the suspected exposure was through aerosolized material after lawn mower or brush cutter use:
- Here is another paper looking at this further:
Introduction and Microbiology
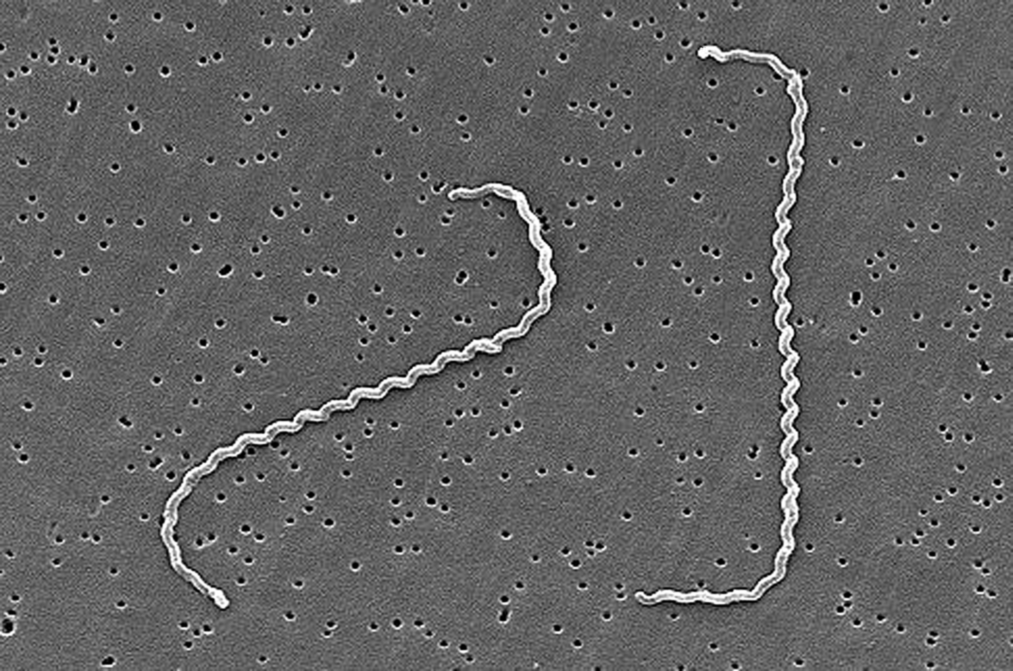
Leptospirosis is caused by infection with pathogenic spirochetes of genus Leptospira
- 22 leptospiral species have been described, and 11 are known to be pathogenic
- Leptospira interrogans, L.kirschneri, L.noguchii are a few of the pathogenic ones
- Isolates are identified by both species and serovar (eg, L.interrogans, icterohemorrhagiae (serogroup), serovar RGA)
- Spiral-shaped highly motile spirochetes with 18+ coils
- Known for a characteristic hook or ‘question mark’ shape at the end of the bacterium
Epidemiology and Transmission
- Leptospirosis is endemic worldwide, but most prevalent in tropical and rural environments
- As discussed on the episode, incidence figures are limited as leptospirosis is underreported
- Overall lower incidence in the US and Canada.
- Hawaii consistently reports the most cases of any state
- Most cases in the US are diagnosed among returning travelers from areas with high infection rates
- Maintained in nature by chronic renal infection of carrier animals (rodents, cattle, pigs, dogs, sheep, goats)
- Most important reservoirs: rodents and small mammals
- Infected animals shed the leptospires in urine, which leads to environmental contamination and persistent, especially water
- Humans are infected incidentally after exposure, usually to animal urine. Human leptospirosis can be acquired via several routes:
- Contaminated water or soil [primary route of transmission]
- Direct contact with animal urine or tissue >> direct mucosal (conjunctival, oral, genital surface, or rarely transplacental) or cutaneous (cuts/abrasions) inoculation
- Aerosol inhalation of microscopic droplets
- Some risk factors to consider:
- Recreational exposure: fresh water swimming, sporting events (like triathlons), canoeing, kayaking
- Occupational exposure: farmers, veterinarians, abattoir workers, lab staff, sewer workers, pet traders, hunters, animal shelter workers
- Rodent infestation >> rats are major source in industrialized areas
- Residence in cities with large population of rodents have been noted to have outbreaks of urban leptospirosis infection
- Walking barefoot in water, contaminated rainwater catchment systems
- Lou Ann mentioned how dynamic weather patterns can play a role in the transmission of zoonotic diseases.
- Here is a paper that looked at mechanisms by which climate change can affect ecologic factors that likely drive an increase in incidence and frequency of leptospirosis outbreaks: Lau CL, Smythe LD, Craig SB, Weinstein P. Climate change, flooding, urbanisation and leptospirosis: fuelling the fire?. Trans R Soc Trop Med Hyg. 2010;104(10):631-638. doi:10.1016/j.trstmh.2010.07.002
- Incidence of hospitalized leptospirosis associated with increased precipitation in Brazil: Hacker KP, Sacramento GA, Cruz JS, et al. Influence of Rainfall on Leptospira Infection and Disease in a Tropical Urban Setting, Brazil. Emerg Infect Dis. 2020;26(2):311-314. doi:10.3201/eid2602.190102
- A look at leptospirosis globally: Costa F, Hagan JE, Calcagno J, et al. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl Trop Dis. 2015;9(9):e0003898. Published 2015 Sep 17. doi:10.1371/journal.pntd.0003898
Clinical Presentations
- Broad range of manifestations
- ~90% of cases are subclinical or mild self-limited disease >> indistinguishable from other febrile illness
- Others present with severe multiorgan disease
- Typical incubation period = 10 days (range 5-14d)
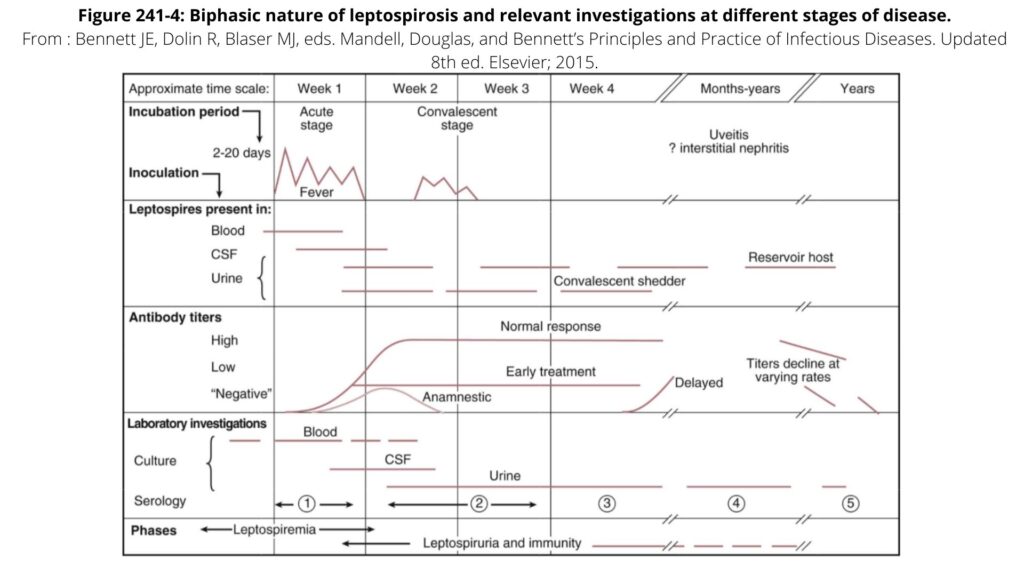
- Severe disease can lead to variable manifestations. Leptospirosis is often described as a biphasic disease with initial septicemic phase, followed by reduction in symptoms, then immune phase with potentially critical illness (more on this below).
- *Many severe cases though can progress directly to fulminant disease*
Acute septicemic stage
High fevers and rigors, headache, anorexia, nausea/vomiting, cough and pharyngitis
Characteristic symptoms:
- *Conjunctival suffusion: extreme conjunctival redness without exudate. Classically described in older literature but only present in ~¼ patients in large series
- *Myalgias/muscle tenderness: severe muscle pain and often prominent in calf and lumbar regions
Lasts about one week and leptospires can be found in blood + CSF
Immune phase
Disappearance of leptospires from blood and CSF coincides with appearance of IgM antibodies
Typical features:
- Acute liver dysfunction with hyperbilirubinemia and jaundice (out of proportion to transaminase elevation)
- Renal failure:
- *Hallmark: nonoliguric hypokalemic renal insufficiency with impaired sodium reabsorption (appears to be due to selective loss of ENaC sodium channel in proximal tubular epithelium)
- Renal biopsy: acute interstitial or immune complex glomerulonephritis
- *Aseptic meningitis in up to 80% patients: intense headache, lymphocytic CSF pleocytosis
- Other CNS manifestations or uveitis are possible
- Pulmonary symptoms: spectrum of pulmonary involvement is broad, but can be serious with diffuse alveolar hemorrhage and acute respiratory distress syndrome
Lasts up to 30 days and leptospires can be detected in urine and other organs
The cardinal features of severe leptospirosis (nonoliguric renal failure, hepatic dysfunction, thrombocytopenia +/- pulmonary involvement) are known by eponym Weil’s disease — which only occurs in about 10% of patients but has high mortality rate
The image below from Mandell gives an overview of leptospirosis as well
Diagnosis is challenging and requires a high index of suspicion based on patient history and epidemiologic exposure!
- Lou Ann mentioned some of the nonspecific but helpful labs you might encounter
- CBC: thrombocytopenia and potentially pancytopenia
- Renal failure with hyponatremia and hypokalemia
- Elevated bilirubin (typically more so than elevated transaminases)
- UA with proteinuria and hematuria
- Leptospires in blood or urine can be directly visualized by dark field microscopy >> but low sensitivity/specificity
- In some rare cases, may grow in culture from blood or urine in special labs – but typically would take 1-2 weeks or longer to grow even with special media
- PCR-based testing of blood, urine, or CSF can be used in acute stage of disease >> but this is often not widely available
- Diagnosis is typically made via serology:
- Not atypical to have negative testing early in course of disease
- Microagglutination test (MAT) is the gold standard or reference assay (should detect agglutinating Ab starting at about 5d after onset)
- Requires live organisms, expertise
- Confirmed case: Most specific if fourfold or greater rise in titer (between acute and convalescent serum)
- Typically single titer of >1:800 is also thought to be suggestive of recent or acute leptospira infection
- Looks at different responses to different serovars
- Not readily available in most places and needs to be sent to CDC
- Other serology testing
- Macroscopic agglutination
- Indirect hemagglutination
- ELISA (in-house often available, but not necessarily validated)
- Notes of caution:
- Our available diagnostics are challenging >> and as new assays develop, they are often compared to our existing reference that also performs poorly. Here is an assessment looking at diagnostic test results in leptospirosis. A combination of blood culture and MAT only had sensitivity of 55% although specificity was >98%
- IgM has possibility of false positive and cross-reactivity with: syphilis, relapsing fever, Lyme, hepatitis, HIV
- IgM after prior leptospirosis infection may be positive for a while >> so use is limited by background seropositivity in endemic areas
Management
- Supportive care with volume repletion and aggressive potassium replacement. In severe disease, patients may require renal replacement therapy and respiratory support
- Many cases are self-limited and do not require antimicrobial therapy
- If ill enough to receive medical attention though, then likely should administer antimicrobials as early in course of disease as suspicion allows (would treat empirically while awaiting results). Guidelines and expert opinion support prompt treatment in suspected or confirmed cases:
- Mild disease can be treated with PO antibiotics:
- Doxycycline 100mg BID x 7d or
- Azithromycin 500mg QD x 3d
- Can use azithro or amoxicillin in pregnancy (azithro a little better if your ddx still includes rickettsial infection)
- In severe cases, would recommend IV therapy with IV Ceftriaxone or IV Penicillin x 7d
- A few papers thinking about treatment in leptospirosis:
- This Cochrane review noted insufficient evidence to recommend in favor of or against antibiotics. Duration of disease did appear shorter among treated patients, but differences were not significant
- Retrospective observational study, delayed initiation of antibiotics (by 2 days or more) was associated with more severe disease
- Trial that compared ceftriaxone (1g daily) with penicillin (1.5 million units q6h) for 7d showed no difference in time to resolution of fever
- Panaphut T, Domrongkitchaiporn S, Vibhagool A, Thinkamrop B, Susaengrat W. Ceftriaxone compared with sodium penicillin g for treatment of severe leptospirosis. Clin Infect Dis. 2003;36(12):1507-1513. doi:10.1086/375226
- Vinetz JM. A mountain out of a molehill: do we treat acute leptospirosis, and if so, with what?. Clin Infect Dis. 2003;36(12):1514-1515. doi:10.1086/375275
- A few notes:
- Antibiotic susceptibility testing is not routinely done due to difficulty, but resistance doesn’t seem to be a frequent issue
- Penicillin and cephalosporins are not active against rickettsiae (which might be on your ddx) >> so doxycycline is often an appropriate choice to cover both leptospirosis and rickettsial infection
- Jarisch-Herxheimer reactions can occur in up to 20% of pts following antimicrobial therapy
- Some suggest steroids or plasmapheresis as adjunct in severe disease, but no clear evidence to justify use
Prevention
- Avoidance potential sources of infection
- Magnitude of risk depends on local prevalence of leptospiral carriage and degree/frequency of exposure
- Reduce
- Direct contact with potentially infected animals
- Indirect contact with urine-contaminated soil and water (e.g. stagnant water, animal farm run-off)
- Protection of food from animal contamination
- Protective measures such as boots, goggles, rubber gloves
- Administration of chemoprophylaxis for individuals with high exposure risk
- Chemoprophylaxis with doxycycline 200mg once a week can be used for individuals who will be unavoidably exposed to endemic environments
- Vaccination
- Animal vaccination can provide some protection >> but immunized animals can become infected and still excrete leptospires in urine
- Human vaccines have been developed for certain circumstances but are not widely available
Other miscellaneous mentions and notes:
- The Boston Magazine article mentioned in the introduction! https://www.bostonmagazine.com/health/2020/01/07/lou-ann-bruno-murtha/
- Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65-97. doi:10.1007/978-3-662-45059-8_5
- Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3(12):757-771. doi:10.1016/s1473-3099(03)00830-2
- Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296-326. doi:10.1128/CMR.14.2.296-326.2001
- A NEJM Clinical-Problem Solving Case: Mixter S, Sedighi Manesh R, Keller SC, Platt L, Hollander H. Spiraling Out of Control. N Engl J Med. 2017;376(22):2183-2188. doi:10.1056/NEJMcps1610072
- Like textbooks?
- Mandell, Principles and Practice of ID, 8th Ed., 9th Ed.:
- Chapter 241: Leptospira Species (Leptospirosis)
- Long, Principles and Practice of Pediatric ID, 5th Ed.:
- Chapter 184: Leptospira
- Comprehensive Review of Infectious Diseases
- AAP Red Book
- Mandell, Principles and Practice of ID, 8th Ed., 9th Ed.:
Episode Art & Infographics
Goal
Listeners will be able to describe the clinical presentation, diagnosis, and management of leptospirosis
Learning Objectives
After listening to this episode, listeners will be able to:
- Recognize leptospirosis as a zoonotic infection that is endemic worldwide and key routes of transmission
- Identify the characteristic symptoms of severe leptospirosis (and Weil’s syndrome)
- Discuss the available diagnostic testing for leptospirosis
- Recommend antimicrobial management for leptospirosis
- Education patients about the prevention strategies for leptospirosis
Disclosures
Our guests (Frances Ue and Lou Ann Bruno-Murtha) as well as Febrile podcast and hosts report no relevant financial disclosures
Citation
Bruno-Murtha, L.A., Ue, F., Dong, S. “#12: Who Framed the Rabbit”. Febrile: A Cultured Podcast. https://player.captivate.fm/episode/527865cc-bb85-464d-861a-37f00cecff83