Table of Contents
Credits
Hosts: Rita Dib, Sara Dong
Guest: Joseph Sassine
Writing: Rita Dib, Joseph Sassine
Producing/Editing/Cover Art/Infographics: Sara Dong
Our Guests
Guest Co-Host
Rita Dib, MD

Rita Wilson Dib is a chief Infectious Diseases fellow at University of Texas Health Science Center (UTHealth) and MD Anderson Cancer Center in Houston, TX, where she is focusing on oncology ID. She previously completed her internal medicine residency at the Medical College of Georgia.
Guest Discussant
Joseph Sassine, MD

Joseph Sassine, MD, FACP is an Assistant Professor of Medicine and Transplant ID Physician at the University of Oklahoma Health Sciences Center, as well as the ID fellowship associate program director. He previously completed his internal medicine residency at the Icahn School of Medicine at Mount Sinai – St. Luke’s-Roosevelt Hospital Center in New York City, and his infectious diseases fellowship at the University of Texas Health Science Center and MD Anderson Cancer Center in Houston. His research interests include viral infections in patients with hematological malignancies, including recipients of hematopoietic cell transplantation and cellular therapies
Consult Notes
Case Summary
47 yo man with DLBCL s/p therapies with R-CHOP, IT chemotherapy, R-DHAP who was found to have recurrent disease and underwent T-cell infusion CARBYKTITM (ciltacabtagene autoleucel) 4 months ago who has RSV pneumonia.
56 yo M with relapsed/refractory multiple myeloma despite four prior lines of therapy who underwent CAR T-cell therapy (BCMA)
Key Points
What are the late complications and infections that may occur beyond day 30 after CAR-T?
Late complications of CAR-T include B-cell aplasia and subsequent hypogammaglobulinemia, prolonged cytopenias, some late neurologic events and immune related effects, and subsequent malignancies such as myelodysplastic syndrome
From an infectious disease perspective, we are most interested in B-cell aplasia with hypogammaglobulinemia and prolonged cytopenias
- Cytopenias initially occurred due to lymphodepleting chemotherapy and prior therapies for the underlying malignancy. They can be observed beyond 30 days post infusion with most products
- In one post hoc analysis of 31 patients with refractory large B-cell lymphoma treated with axi-cel, a CD19 product at MD Anderson, 48% had grade 3 through 4 cytopenia by day 30, 29% had neutropenia
- On univariate analysis, performance status, more than 3 prior therapies and low ALC at baseline were the most important risk factors
- Among patients with ongoing remission, 27% had grade 3-4 cytopenias at 1 year, and 11% at 2 years
- All of the patients recovered CD8 T cells and CD56 NK cells at 1 year. Only two thirds of the patients recovered their CD4 T cells at 1 year and 71% recovered them at 2 years.
- Strati P, Varma A, Adkins S, et al. Hematopoietic recovery and immune reconstitution after axicabtagene ciloleucel in patients with large B-cell lymphoma. Haematologica. 2021;106(10):2667-2672. Published 2021 Oct 1. doi:10.3324/haematol.2020.254045
- In one post hoc analysis of 31 patients with refractory large B-cell lymphoma treated with axi-cel, a CD19 product at MD Anderson, 48% had grade 3 through 4 cytopenia by day 30, 29% had neutropenia
- B-cell aplasia is an on-target, off-tumor effect of CD19 CAR-T
- CD19 is expressed on the B-cell lineage from pre-B cells through memory B cells
- CD19 CAR-T does not discriminate between the malignant B cells and the healthy B cells, and this will lead to B-cell aplasia
- This will affect more than 85% of the patients in the first month after CD19 CAR-T, and will persist in about 40 to 60% of the patients at the 1-year mark
- This constitutes the major risk factor for hypogammaglobulinemia after CAR-T
- Prior treatments before CAR-T, the nature of the hematological malignancy, and other post CAR-T acute toxicities requiring additional immunosuppression can also contribute to worsening hypogammaglobulinemia.
- Kampouri E, Walti CS, Gauthier J, Hill JA. Managing hypogammaglobulinemia in patients treated with CAR-T-cell therapy: key points for clinicians. Expert Rev Hematol. 2022;15(4):305-320. doi:10.1080/17474086.2022.2063833
- Some data:
- Logue JM, Zucchetti E, Bachmeier CA, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica. 2021;106(4):978-986. Published 2021 Apr 1. doi:10.3324/haematol.2019.238634 → A study of 85 adults with large B-cell lymphoma who got axi-cel, 28% of them had hypogammaglobinemia prior to lymphodepletion, IgG levels reached the nadir at 6 months, at 1 year about half of the patients did not need immunoglobulin replacement therapy.
- Baird JH, Epstein DJ, Tamaresis JS, et al. Immune reconstitution and infectious complications following axicabtagene ciloleucel therapy for large B-cell lymphoma. Blood Adv. 2021;5(1):143-155. doi:10.1182/bloodadvances.2020002732 → Another study of 41 patients with large B-cell lymphoma who also got axi-cel, 10% had hypogammaglobulinemia prior to lymphodepletion, over the first 9 months 62% had hypogammaglobulinemia and 37% received immunoglobulin replacement therapy.
- It seems that lower rates have been reported with other CD19 products, 14% in the liso-cel phase 1/2 trial for large B-cell lymphoma and 1% for the brex-cel phase 2 trial for mantle cell lymphoma. Keep in mind that these are clinical trials so the inclusion criteria are stricter than review of data.
- As you noticed, this is mostly about IgG. There is very scarce data when it comes to IgA and IgM.
The majority of late infections after CD19 CAR-T tend to be viral
- In ep 73, we discussed the first study of infections after CD19 CAR-T out of Fred Hutch
- A follow-up of 86 patients from that cohort who stayed alive for at least 1 year showed that 16% of these patients had prolonged cytopenias, lasting 15 to 22 months
- Two thirds of the patients had hypogammaglobulinemia beyond day 90, 64% of these patients had hypogammaglobulinemia prior to CAR-T to begin with, and were more likely to have ALL or CLL as compared to non-Hodgkin’s lymphoma.
- The overwhelming majority of the infections were upper respiratory infections, followed by lower respiratory infections.
- Almost all the upper respiratory infections were managed as an outpatient, the lower respiratory infections were managed equally between outpatient and inpatient.
- Of the other infections that occurred 50% of these were skin infections.
- Overall, 20% of the patients required inpatient admission and only 5% required ICU admission.
- Cordeiro A, Bezerra ED, Hirayama AV, et al. Late Events after Treatment with CD19-Targeted Chimeric Antigen Receptor Modified T Cells. Biol Blood Marrow Transplant. 2020;26(1):26-33. doi:10.1016/j.bbmt.2019.08.003
- Another cohort covered in ep 73 of CD19 CAR-T cell recipients at Sloan-Kettering
- Showed beyond day 30, 54% of the patients developed a bacterial infection, 44% developed a viral infection
- Of those, 75% were respiratory viral infections, 1 patient had a CMV viremia, 2 patients had VZV breakthrough on acyclovir, and 1 patient had pneumocystis 4 months after stopping pentamidine and 9 months after CAR-T
- In that cohort both the IgG level and the CD4 count reached a nadir at about 9 months then started increasing
- The major predictor for viral infection was a low IgG level prior to lymphodepletion.
- Wudhikarn K, Palomba ML, Pennisi M, et al. Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma. Blood Cancer J. 2020;10(8):79. Published 2020 Aug 5. doi:10.1038/s41408-020-00346-7
- Showed beyond day 30, 54% of the patients developed a bacterial infection, 44% developed a viral infection
- One more cohort covered in ep 73 as well was out of Moffitt, also CD19 CAR-T
- After day 30, 44% of the patients had an infection. 59% of these were respiratory viruses. 22% of the patients had a severe infection, there were 5 pneumonias, 1 MRSA bacteremia and 1 neutropenic fever.
- There were no significant differences in CD4, CD8, or IgG levels at the therapy between patients who developed infections after Day 30 and those who did not.
- Logue JM, Zucchetti E, Bachmeier CA, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica. 2021;106(4):978-986. Published 2021 Apr 1. doi:10.3324/haematol.2019.238634
In the episode, there was a quick focus on respiratory viral infections
Our co-host Rita had recently published: Wilson Dib R, Ariza-Heredia E, Spallone A, Chemaly RF. Respiratory Viral Infections in Recipients of Cellular Therapies: A Review of Incidence, Outcomes, Treatment, and Prevention. Open Forum Infect Dis. 2023;10(4):ofad166. Published 2023 Mar 25. doi:10.1093/ofid/ofad166
Joseph discussed how most of the data on respiratory viral infections after CAR-T was generated from evaluation of the major CAR-T trials:
- In the first 30 days after CAR-T, other rates of respiratory viral infections ranged between 8 and 20%
- Those rates were significantly higher later on, reaching 58% between day 13 and day 90, and 53% within 1 year
- This is likely due to a combination of the long-term B-cell aplasia and hypogammaglobinemia which we just discussed, as well as patients trying to reengage with their communities after finishing the more intensive part of the treatment
- It seems that the most common respiratory viruses in the community are also the most common respiratory viruses affecting recipients of cellular therapies, with rhinovirus taking the lead and accounting for about a third of the infections, influenza for about the fifth of infections and parainfluenza for about 15%.
- There certainly is a high risk for severe disease and higher mortality due to respiratory viral infections in these patients
- In terms of risk stratification, we do not have a specific or tangible tool to use.
- There has been an immunodeficiency scoring index which was initially developed by Dr. Roy Chemaly to assess the risk for progression from URI to LRI and mortality in allogeneic HCT recipients with an RSV infection
- This ISI score has been validated for other respiratory viral infections in this patient population
- ISI assigns a weighted score for different risk factors including neutropenia, lymphopenia, age, corticosteroids, recent allogeneic HCT, and those factors can probably be used to come up with a similar score in CAR-T cell recipients
- The 2 additional factors in ISI, myeloablative conditioning regimen and GVHD, would not be applicable for CAR-T cell recipients.
- Shah DP, Ghantoji SS, Ariza-Heredia EJ, et al. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood. 2014;123(21):3263-3268. doi:10.1182/blood-2013-12-541359
The episode featured findings consistent with profound B-cell aplasia and severe hypogammaglobulinemia with a current episode of RSV with likely pneumonia.
- Consideration of oral ribavirin was discussed
- Traditionally aerosolized ribavirin has been used, but this is very cumbersome to administer and comes with high risk to the patient and staff
- Oral ribavirin has more recent data suggesting similar efficacy with better tolerability and safety
- One could also consider intravenous immunoglobulin given the presence of lower respiratory tract involvement in the example case
- There have been no randomized control trials comparing ribavirin with IVIG to ribavirin alone and retrospective analyses have shown variable results for the combination, which is probably due to the fact that humanized IVIG lacks sufficient specific antibodies to RSV, hence the mixed response results
- Another question that comes up often on consults is the use of palivizumab which is an RSV specific monoclonal antibody that is really only approved for primary prophylaxis and high-risk neonates in children
- Experience using it for treatment is very limited and no recommendation can be made based on the available data
- Joseph mentioned that the ASTCT ID group has developed guidelines for management of RSV and these should hopefully be out soon.
- Khawaja F, Chemaly RF. Respiratory syncytial virus in hematopoietic cell transplant recipients and patients with hematologic malignancies. Haematologica. 2019;104(7):1322-1331. doi:10.3324/haematol.2018.215152
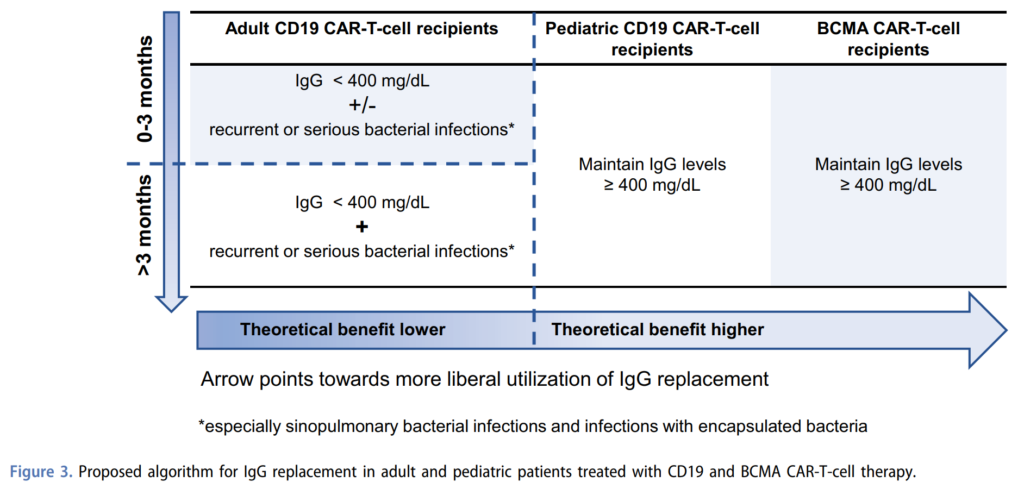
- Next Joseph discussed management of hypogammaglobulinemia. He described 3 factors to take into consideration when making the decision for IgG replacement:
- 1. The extent of hypogammaglobulinemia, as in the actual serum IgG level, and 400 mg/dL is the most commonly used cutoff
- 2. The occurrence and frequency of infections, particularly serious or recurrent infections, especially bacterial infections with encapsulated organisms
- 3. How far out from CAR-T the patient is, would be more liberal in administering immunoglobulin replacement therapy in patients who are less than 3 months out, compared to patients with received CAR-T more than 3 months ago.
- Joseph recommended this expert review from the group at Fred Hutch, where they provide a framework for immunoglobulin replacement therapy
- In the first 3 months after CD19 CAR-T, IgG level less than 400 mg/dL plus or minus recurrent or serious bacterial infections would be a good indication
- Beyond 3 months, they suggest the combination of both hypogammaglobulinemia and recurrent or serious bacterial infections, with emphasis on sinopulmonary bacterial infections and infections with encapsulated bacteria
- Kampouri E, Walti CS, Gauthier J, Hill JA. Managing hypogammaglobulinemia in patients treated with CAR-T-cell therapy: key points for clinicians. Expert Rev Hematol. 2022;15(4):305-320. doi:10.1080/17474086.2022.2063833
- Hill JA, Giralt S, Torgerson TR, Lazarus HM. CAR-T – and a side order of IgG, to go? – Immunoglobulin replacement in patients receiving CAR-T cell therapy. Blood Rev. 2019;38:100596. doi:10.1016/j.blre.2019.100596
Vaccinations after CAR-T
- Vaccinations after CAR-T are an essential cornerstone in preventing infections in this patient population, but it remains unclear who, when and how to vaccinate these patients
- Understanding response to vaccines after CAR-T is still the subject of ongoing studies, especially that different vaccines have different levels of immunogenicity.
- It is important that, prior to CAR-T, a comprehensive vaccination history is obtained, including for influenza and pneumococcal vaccines
- Prior to CAR-T, the 2 vaccines that should get administered are:
- Annual influenza vaccine during influenza season, at least 2 weeks prior to lymphodepletion, and
- A complete series of SARS-CoV-2 vaccines if there is time (otherwise the full series can be administered at least 3 to 6 months after CAR-T)
- Additional vaccines are usually not recommended prior to CAR-T, given that most of these patients have relapsed/refractory malignancies, have received extensive antitumor therapies, and we would expect a lower immunological response.
- After CAR-T, you need to make a distinction between killed/inactivated vaccines and live/non-live adjuvant vaccines
- Killed/inactivated vaccines can be administered if the patient is in remission, 6 months or more post CAR-T, 2 months or more after the last IVIG, and not receiving chemotherapy or other immunosuppressive therapies that would affect T or B-cell function
- Those vaccines will include the pneumococcal conjugate vaccine, Haemophilus influenza type b, hepatitis A and B, diphtheria/tetanus/acellular pertussis vaccine (DTaP preferred over Tdap)
- Titers are usually checked after the first dose, and for those patients with seroprotective titers and no history of HCT, no additional vaccines are needed
- Otherwise, additional doses of the vaccines are required to complete a primary series
- Joseph touched on how different centers are trying to develop their strategy around PCV20 and PCV 15 now that these are available
- Additional killed/inactivated vaccines such as meningococcal vaccine, inactivated polio and HPV are typically administered past 18 months and those patients will have responded to the initial vaccine series.
- Live and non-live adjuvant vaccines can be administered when the patient is in remission, more than 1 year post CAR-T, more than 2 years post HCT and meeting other post-HCT criteria, more than 1 year after immunosuppressive therapies and more than 8 months after the last IVIG, a CD4 count > 200 is also required
- These would include MMR, and the recombinant zoster vaccine which is now approved for all adult immunocompromised patients regardless of age
- Killed/inactivated vaccines can be administered if the patient is in remission, 6 months or more post CAR-T, 2 months or more after the last IVIG, and not receiving chemotherapy or other immunosuppressive therapies that would affect T or B-cell function
- Hill JA, Seo SK. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood. 2020 Aug 20;136(8):925-935. doi: 10.1182/blood.2019004000. PMID: 32582924; PMCID: PMC7441168.
How is BCMA CAR T different from CD19 CAR T in terms of risk factors for infection and infectious complications?
- BCMA CAR-T are relatively recent in use compared to CD19 CAR-T, so our understanding of the immunological dysfunctions and subsequent infections that occur after BCMA CAR-T is currently evolving
- BCMA CAR-T is used in patients with relapsed refractory multiple myeloma, and due to the nature of the disease and the therapies used to treat it, the depletion of long-lived plasma cells occurs before BCMA CAR-T, and continues after CAR-T which targets the BCMA antigen present on long-lived plasma cells
- Hypogammaglobulinemia as a result of B-cell depletion in the setting of on-target/off-tumor effects of CD19 CAR-T are observed. This hypogammaglobulinemia is even more important after BCMA CAR-T on 2 levels:
- A larger proportion of patients get to CAR-T with pre-existing hypogammaglobulinemia
- A study of 55 adults who have received BCMA CAR-T for multiple myeloma had 42% of the patients with an IgG level less than 300 mg/dL prior to liver depletion
- This gets accentuated after CAR-T, and reaches 70% in the first 3 months, and decreases only to 41% at the 1-year mark.
- Kambhampati S, Sheng Y, Huang CY, et al. Infectious complications in patients with relapsed refractory multiple myeloma after BCMA CAR T-cell therapy. Blood Adv. 2022;6(7):2045-2054. doi:10.1182/bloodadvances.2020004079
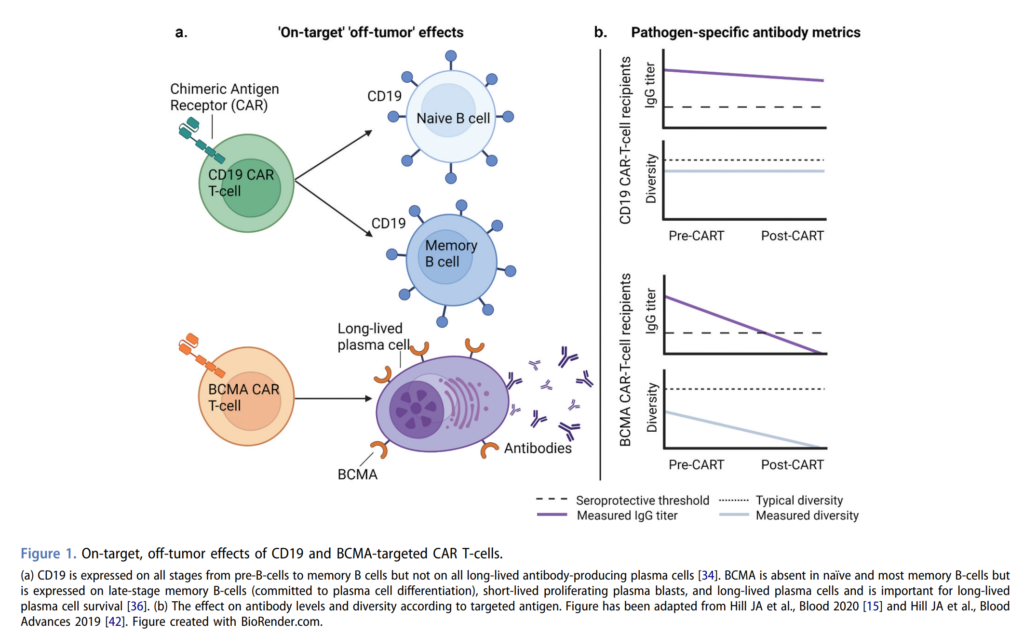
- In patients receiving CD19 CAR-T, the main hypogammaglobulinemia problem is an overall low serum IgG level
- When you look at pathogen specific antibodies, and the CD19 CAR-T population, these are overall comparable to population-based cerebral venous data and do not correlate with total IgG
- There might be some protection with lower serum prevalence for Streptococcus pneumoniae and Haemophilus influenza type b which gets inadvertent compensation by using trimethoprim/sulfamethoxazole for pneumocystis prophylaxis
- This is due to the fact that CD19 is expressed on pre-B cells all the way through memory B cells but not on the long-lived antibody producing plasma cells
- As such after CD19 CAR-T, the CD19 negative BCMA positive plasma cells remain, whereas the CD19 positive B cells and plasma cells are eliminated, causing this discrepancy between total and pathogenic specific antibody levels.
- A larger proportion of patients get to CAR-T with pre-existing hypogammaglobulinemia
- On the other side, in BCMA CAR-T, in addition to overall hypogammaglobulinemia, pathogen specific IgG levels are significantly lower than after CD19 CAR-T
- This is due to the fact that BCMA is expressed on late stage memory B cells and long-lived plasma cells and is important for the survival of those long-lived plasma cells
- After BCMA CAR-T, all plasma cells populations are depleted thus resulting in more severe antibody deficiencies, and both total and pathogen specific antibodies.
- Walti CS, Krantz EM, Maalouf J, et al. Antibodies against vaccine-preventable infections after CAR-T cell therapy for B cell malignancies. JCI Insight. 2021;6(11):e146743. Published 2021 Jun 8. doi:10.1172/jci.insight.146743
- As a side note, in the pediatric population, for whom only CD19 CAR-T are approved for B-ALL, those patients have limited preestablished plasma cells to begin with before undergoing CD19 CAR-T, and thus will only have limited pathogen specific antibody titers and spectra after CD19 CAR-T
- For this reason, the expert recommendation for intravenous immunoglobulin replacement tends to be more liberal for BCMA CAR-T cell recipients and pediatric CD19 CAR-T cell recipients as compared to adults in 19 CAR-T cell recipients
- And for these two special populations, the recommendation would be to maintain IgG levels greater than 400 mg/dL and the theoretical benefit of a more liberal utilization of IgG replacement is higher.
- Kampouri E, Walti CS, Gauthier J, Hill JA. Managing hypogammaglobulinemia in patients treated with CAR-T-cell therapy: key points for clinicians. Expert Rev Hematol. 2022;15(4):305-320. doi:10.1080/17474086.2022.2063833
- Now looking at infections after BCMA CAR-T, the first study was published in 2022 out of Fred Hutch: Josyula S, Pont MJ, Dasgupta S, et al. Pathogen-Specific Humoral Immunity and Infections in B Cell Maturation Antigen-Directed Chimeric Antigen Receptor T Cell Therapy Recipients with Multiple Myeloma. Transplant Cell Ther. 2022;28(6):304.e1-304.e9. doi:10.1016/j.jtct.2022.03.005
- Looked at 32 adults who are enrolled in phase 1 trials of BCMA CAR-T, 10 of whom received a gamma secretase inhibitor on top of lymphodepleting chemotherapy, and everyone had an antimicrobial prophylaxis regimen
- Before CAR-T, 81% of the patients lacked serum protective levels of measles IgG
- After CAR-T, the only patients who retained a serum protective level had non-IgG myeloma
- Additionally, IgG multiple myeloma had significantly fewer viral epitope hits compared to non-IgG myeloma, both before and after CAR-T
- So, patients with IgG multiple myeloma may be at the particularly increased risk for infection compared to the other patients with multiple myeloma
- In comparison: Hill JA, Krantz EM, Hay KA, et al. Durable preservation of antiviral antibodies after CD19-directed chimeric antigen receptor T-cell immunotherapy. Blood Adv. 2019;3(22):3590-3601. doi:10.1182/bloodadvances.2019000717
- A study of 22 CD19 CAR T recipients by the same group showed that only 1 patient lost measles seroprotection after CD19 CAR T and that patient had a prior allogeneic HCT.
- In terms of infections, the first 28 days, there was about the same proportion of bacterial and viral infections, and beyond that most infections were viral.
- Another cohort, this time from UCSF: Kambhampati S, Sheng Y, Huang CY, et al. Infectious complications in patients with relapsed refractory multiple myeloma after BCMA CAR T-cell therapy. Blood Adv. 2022;6(7):2045-2054. doi:10.1182/bloodadvances.2020004079
- 55 patients treated with BCMA CAR-T” for up to 1 year, all of whom also received antimicrobial prophylaxis
- In this cohort, the highest incidence of infection was actually noticed between day 31 and day 100, within almost equal distribution between bacterial and viral infections during that time
- Beyond day 100, there were more viral infections but the rate of bacterial infections was still decent.
- A direct comparison of this cohort with another group at UCSF: Infectious Complications of BCMA-Targeted and CD19-Targeted Chimeric Antigen Receptor T-Cell Immunotherapy | Blood | American Society of Hematology (ashpublications.org)
- 49 patients with non-Hodgkin’s lymphoma treated with CD19 CAR-T at UCSF
- This comparison shows a significantly higher rate of bacterial infections in patients with CD19 CAR-T, 73% versus 40%, but a significantly higher risk of viral infections in the BCMA CAR-T group 53% versus 20%.
- Patients with CD19 CAR-T tended to have more severe infections, 18% versus 5%.
- There were more bloodstream infections 50% versus 2% and more GI infections 10% versus none in the CD19 CAR-T versus the BCMA CAR-T group.
- The risk factors for infection were identified as use of steroids and post CAR-T hypogammaglobulinemia with an IgG level less than 600 mg/dL.
How often do these patients develop CMV infection and disease? What are the risk factors?
- Some of the previous cohorts described have reported occurrence of CMV infection (meaning CMV viremia) and there are scattered case reports of CMV end organ disease
- The challenge is that unlike allogeneic HCT, CMV PCR surveillance is not routinely performed – so cases of CMV reactivation wouldn’t really get diagnosed until they reach end-organ disease stage
- This was reported in an IDWeek 2021 abstract led by Joseph: 930. Clinically Significant CMV Infections in Patients with Lymphoma or Multiple Myeloma | Open Forum Infectious Diseases | Oxford Academic (oup.com)
- These patients had lymphoma or myeloma and developed clinically significant CMV infection, the fifth of them actually had CAR-T cell, and about two thirds of the patient’s actually had CMV end organ disease on initial presentation. The all-cause mortality was 55% at day 100 and CMV related mortality was 11% among patients with end organ disease. So, this is a population that has been classically unrecognized to have a decent burden of CMV infection and disease.
- Joseph also looked at this during a project he worked on in fellowship, which was presented at ASH 2022: Cytomegalovirus (CMV) Reactivation within in the First Year after Chimeric Antigen Receptor (CAR) T Cell Therapy: Experience from the First Two Years at a Major Cancer Center | Blood | American Society of Hematology (ashpublications.org)
- Among 230 patients who received CAR-T cells, 10% developed clinically significant CMV infection, which again is defined as CMV disease or CMV infection requiring antiviral therapy. Clinically significant CMV infections were identified at a median of 17 days after CAR-T cell, and as expected there was a high rate of end organ disease with about a third of these patients diagnosed with CMV pneumonia. Of the patients who were diagnosed with clinically significant CMV infection, 55% passed away within 60 days of the diagnosis. Patients with clinically significant CMV infection had a higher overall mortality rate after 1-year post CAR-T compared to patients who did not have clinically significant CMV infection. The risk factors were identified to be low ANC at day 30, grade 2 or higher CRS or ICANS, requiring treatment for CRS or ICANS, and a high cumulative steroid dose during the first 30 days after CAR-T cell infusion
- Another study mentioned: CMV and HHV-6 after Chimeric Antigen Receptor (CAR)-T-Cell Immunotherapy for B-Cell Malignancies: A Prospective Study – Transplantation and Cellular Therapy, Official Publication of the American Society for Transplantation and Cellular Therapy (astctjournal.org)
- Enrolled 36 CMV seropositive adult CAR-T recipients and looked at CMV and HHV-6 reactivation
- ~20% of patients had CMV reactivation; ~10% had HHV-6 reactivation
- Of those 7 patients with CMV reactivation, only 2 received preemptive therapy and there were no cases of end organ disease for either CMV or HHV-6
- So some centers are moving towards monitoring for CMV in high risk patients, such as those with persistent cytopenias, those with high grade CRS or ICANS, those requiring high dose steroids for treatment of HLH/MAS
- We still need a better understanding of patients at high risk to target for monitoring, preemptive therapy, or even prophylaxis as well as how CMV specific immune reconstitution occurs after CAR-T
What are the risk factors for invasive fungal infections in this population?
- There probably is not a uniform answer to this across the US, but there have been multiple reports of IFI in this patient population
- Pneumocystis:
- Many experts knew that these patients were at increased risk for PCP and most, if not all of the cohorts discussed, including cohorts that enrolled patients during clinical trials, had PCP prophylaxis in place
- There have not been many reports of pneumocystis infection in these patients
- An extensive review of fungal infections published in 2021 was able to identify only 3 cases (2 of these had already completed prophylaxis; one was nonadherent): Little JS, Weiss ZF, Hammond SP. Invasive Fungal Infections and Targeted Therapies in Hematological Malignancies. J Fungi (Basel). 2021;7(12):1058. Published 2021 Dec 10. doi:10.3390/jof7121058
- Candida
- The early period after CAR-T which is characterized by neutropenia, mucositis, central venous catheters, ICU admission, is probably the highest risk for Candida infections → most centers have adopted Candida prophylaxis during periods of neutropenia
- Mold
- Joseph described 2 important risk factors for invasive mold infection in post-CAR-T period:
- 1. Prolonged neutropenia
- 2. Long courses of steroids (and other immunosuppressants) for CRS, but particularly for ICANS, which is the same immunological problem seen in allogeneic HCT recipients who develop GVHD and require long courses of steroids. The Little, et al. paper above calls it a GVHD-like phenotype
- These two groups of patients are candidates for mold prophylaxis
- It can be complicated or nuanced based on your geographical location
- Little JS, Aleissa MM, Beluch K, et al. Low incidence of invasive fungal disease following CD19 chimeric antigen receptor T-cell therapy for non-Hodgkin lymphoma. Blood Adv. 2022;6(16):4821-4830. doi:10.1182/bloodadvances.2022007474 → study of 280 patients receiving CD19 CAR-T cell for non-Hodgkin’s lymphoma at Dana-Farber in Boston showed extremely low rates of IFI with no routine yeast or mold prophylaxis
- In contrast, Joseph described how he and Rita encountered invasive mold infections in patients with hematological malignancies on a daily basis and do give mold active prophylaxis for high risk patients
- In different geographical locations with different climates, different soil composition, different humidity, the decision to administer mold active prophylaxis might be more nuanced, and you would need to consider the risks associated with these drugs, including adverse events and drug interactions.
- Joseph described 2 important risk factors for invasive mold infection in post-CAR-T period:
Infographics
Goal
Listeners will be able to understand late complications after CAR-T therapy
Learning Objectives
After listening to this episode, listeners will be able to:
- Describe late complications (beyond 30 days) of CAR-T therapy such as prolonged cytopenias and hypogammaglobulinemia
- Discuss approach to vaccination after CAR-T
- Compare BCMA CAR-T to CD19 CAR-T in terms of risk factors for infection
Disclosures
Our guests as well as Febrile podcast and hosts report no relevant financial disclosures.
As Joseph noted on the audio, this episode discusses commercially available, FDA-approved products (there are a broad range of other cellular therapy products that are still in investigation for a wide variety of conditions/indications)
Citation
Sassine, J., Dib, R., Dong, S. “#74: 2 Fast 2 Feverish: Late ID Complications of CAR-T therapy”. Febrile: A Cultured Podcast. https://player.captivate.fm/episode/288fa311-9918-4cca-b58e-307ad3ac1e69